Impact of Carbon-Coated Aluminum Foil on LiFePO4 Electrode Conductivity Performance
Lithium-ion batteries are a multi-component integrated system composed of positive and negative electrode sheets, separators, electrolytes, and other components. Among them, the positive and negative electrode are important components that provide and influence battery performance. During battery operation, electrons and ions are transported within the electrode, undergoing a series of chemical and electrochemical reactions. Therefore, the conductivity and uniformity of the conductive network in the electrode are important factors affecting battery performance. The current collector, serving as a carrier for conducting electrons and supporting active materials inside lithium-ion batteries, plays a crucial role in the final performance of the cell.
Aluminum foil is the most used positive electrode current collector. To improve the rate capability, cycling stability, and service life of the electrode, some conductive coatings are applied to the surface of the aluminum foil. This can effectively reduce the interface contact resistance between the current collector and active particles, increase the bonding strength between active materials and the current collector, and minimize issues such as active particle detachment during electrode cycling[1].
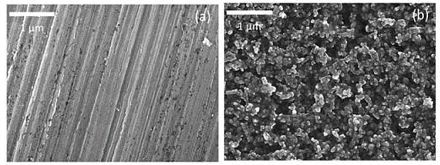
Figure 1. Schematic diagram of surface morphology for bare aluminum foil and carbon-coated aluminum foil [1]
Currently, most of the carbon-coated aluminum foils are utilized LiFePO4 power batteries. This is mainly due to the inherent low electronic and ionic conductivity of the olivine crystal structure LiFePO4 material and issues such as weak adhesion to the current collector[2]. However, the application of carbon-coated aluminum foil is somewhat limited. Carbon-coated aluminum foil exhibits lower contact impedance and higher adhesion. When applied to the positive electrode current collector of LiFePO4 , it reduces the charge transfer resistance between them and the internal resistance of the battery. This diminishes internal polarization of the battery, enhances the diffusion rate of lithium-ion within the material, thereby improving the rate capability and cycling performance of the battery[3-5].
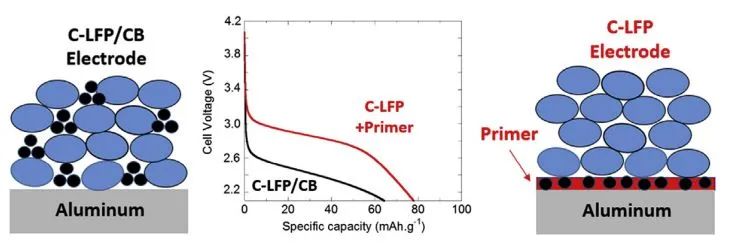
Figure 2. Schematic Diagram Illustrating the Impact of Carbon-Coated Current Collectors on Cell Performance[1]
This study primarily utilizes IEST Battery Electrode Resistance Analyzer (BER2500) to comparatively evaluate the electronic conductivity of bare aluminum foil and carbon-coated aluminum foil, as well as the electronic conductivity of LiFePO4 electrodes coated on bare aluminum foil versus those coated on carbon-coated aluminum foil. The aim is to investigate the improvement in electronic conductivity of LiFePO4 electrodes facilitated by carbon-coated aluminum foil.
1. Test Conditions
1.1 Test Equipment
Figure 3 shows the Battery Electrode Resistance Analyzer (BER2500) independently developed by IEST. The electrode sample has a diameter of 14mm and can withstand pressure in the range of 5 to 60 MPa. It can simultaneously collect parameters such as electrode resistance, resistivity, conductivity, and compacted density. The equipment is depicted in Figures 3(a) and 3(b).
Figure 3. (a): External view of BER2500; (b): Structural diagram of BER2500
1.2 Experimental Procedure
(1) Foil Resistance Test
① Prepare a set of bare aluminum foil and carbon-coated aluminum foil, denoted as KB and TCB respectively.
② Set the test parameters on the MRMS software, select the single-point test mode, with a pressure of 5 MPa and a holding time of 15 seconds. Take 8 data samples for each foil, and the software automatically reads data such as foil thickness, resistance, resistivity, conductivity, etc.
(2) Electrode Resistance Test
① Prepare a set of LiFePO4 electrodes coated on bare aluminum foil and LiFePO4 electrodes coated on carbon-coated aluminum foil, denoted as K-LFP and TC-LFP respectively.
② Set the test parameters on the MRMS software, select the single-point test mode, with a pressure of 25 MPa and a holding time of 15 seconds. Take 8 data samples for each electrode, and the software automatically reads data such as electrode thickness, resistance, resistivity, conductivity, etc.
2. Data Analysis
2.1 Foil Resistance Analysis
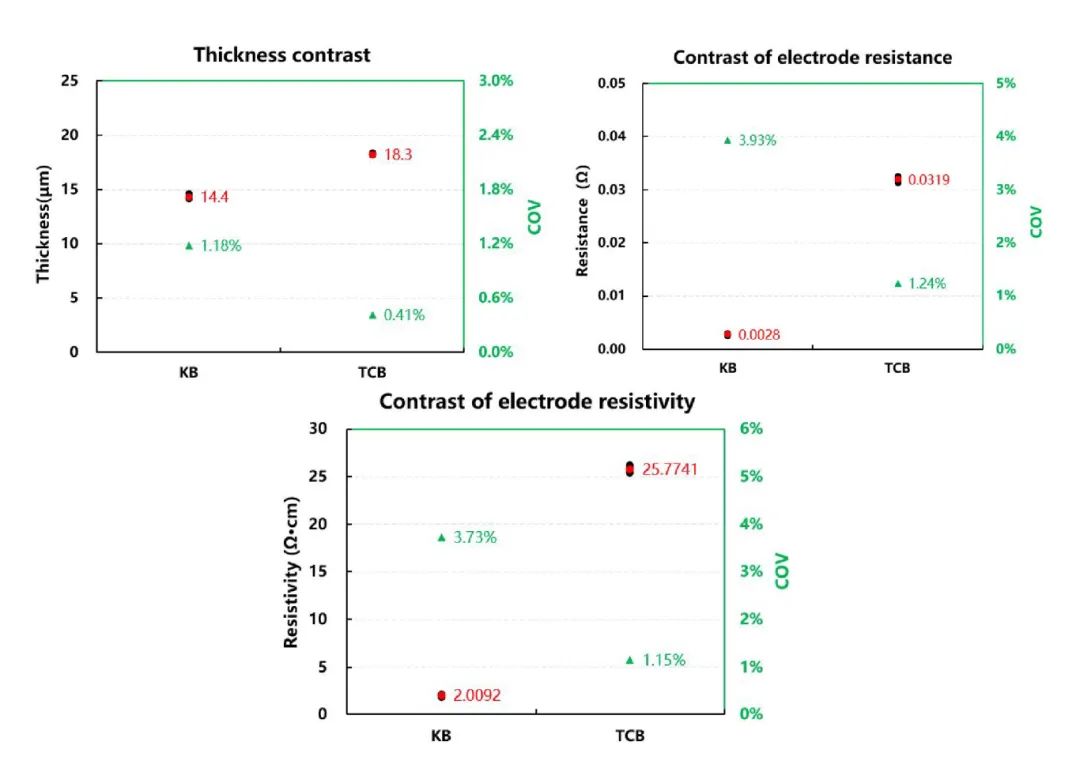
Figure 4. Comparison of Thickness, Resistance, and Resistivity Test Results for Two Types of Foils
Figure 4 presents a comparative analysis of the test results for thickness, resistance, and resistivity of two types of foils. From the thickness data, it can be observed that the carbon layer on the carbon-coated aluminum foil is double-sided, resulting in a thickness of approximately 2µm for the conductive carbon layer. Comparing the resistance and resistivity of these two types of foils: bare aluminum foil < carbon-coated aluminum foil, indicating that adding a carbon coating to the current collector reduces the conductivity of the foil.
This is mainly because the introduction of the carbon coating increases the contact resistance between the carbon coating itself and the foil during the two-probe principle test of foil resistance, weakening the conductivity of electrons. Therefore, the resistance and resistivity of carbon-coated aluminum foil are higher than those of bare foil. Comparing the coefficient of variation (COV) values for resistance and resistivity testing, the stability of resistance testing for foils with a carbon coating is better than that of bare aluminum foil. This is because the carbon coating increases the surface roughness of the aluminum foil, providing more electron pathways, resulting in more stable test data.
2.2 Electrode Resistance Analysis
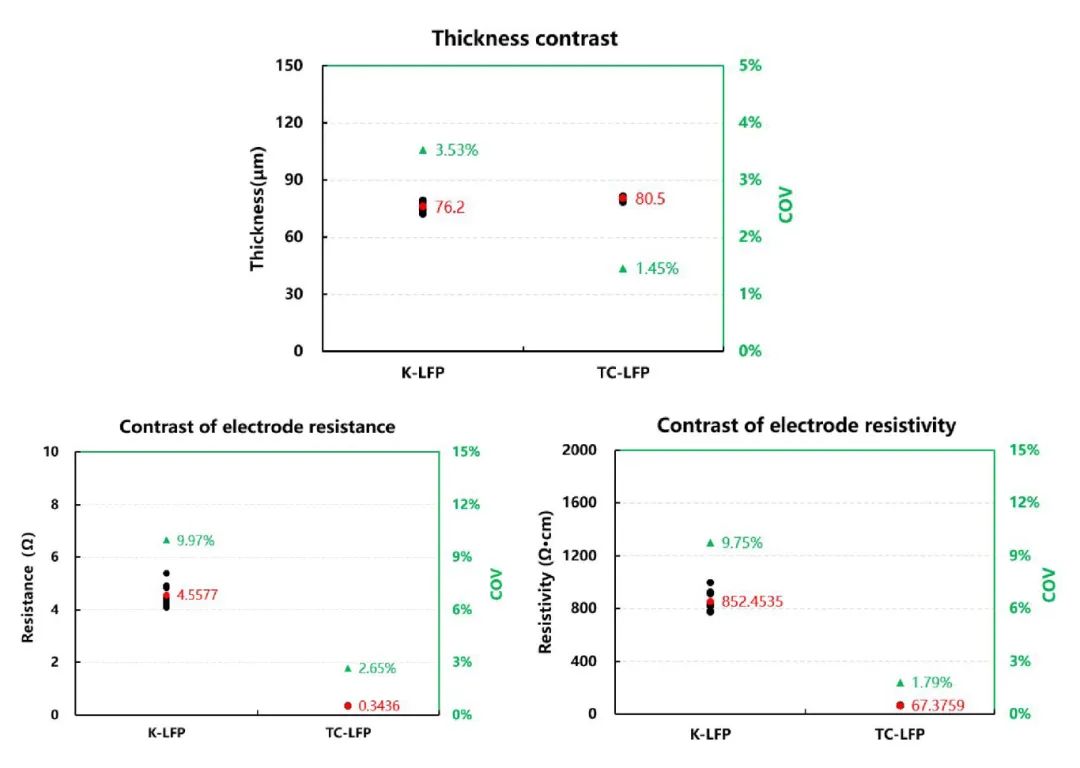
Figure 5. Comparison of Thickness, Resistance, and Resistivity Test Results for Two Types of Electrodes
From the comparative test results of the resistance and resistivity of the two types of electrodes in Figure 5, it can be observed that although the resistance of the carbon-coated aluminum foil is higher than that of the bare aluminum foil, the electrode resistance and resistivity of LiFePO4 electrode coated with carbon-coated aluminum foil are lower than those of the LiFePO4 electrode using bare aluminum foil after applying active materials. Additionally, the stability of the test data is better. This is mainly because the conductive carbon layer on the carbon-coated aluminum foil can increase the contact area between LiFePO4 and the current collector, enabling the collection of more micro-currents from LiFePO4 and providing excellent static conductivity.
As a result, the contact resistance between LiFePO4 and the current collector is significantly reduced. This allows for faster electron transmission and current collection throughout the battery, thereby improving the rate capability of the battery during charging and discharging cycles. Moreover, the carbon coating also contains a small amount of binder, which enhances the flexibility and mechanical buffering of the LiFePO4 electrode. This strengthens the adhesion between LiFePO4 and the current collector, minimizing the loss of contact area caused by stress generated at the interface during long-term cycling of the battery.
3. Summary
This study utilized the Battery Electrode Resistance Analyzer (BER2500) developed by IEST to test the electronic conductivity performance of bare aluminum foil and carbon-coated aluminum foil, as well as the electronic conductivity performance of LiFePO4 electrodes coated on bare aluminum foil and carbon-coated aluminum foil. The study investigated the impact of adding a carbon coating on the electronic conductivity of foils and LiFePO4 electrodes.
Although using carbon-coated aluminum foil increases the resistance and resistivity of the foil, it improves the uniformity and consistency of electronic conductivity. After applying LiFePO4, the resistance and resistivity of the LiFePO4 electrodes are reduced when using carbon-coated aluminum foil, and the stability of the test data is enhanced. This effectively improves the internal resistance of LiFePO4 batteries.
In summary, in addition to improving interface contact resistance, the use of carbon-coated aluminum foil also potentially provides the following synergistic benefits:
(1) The chemically and electrochemically stable conductive layer can serve as an effective diffusion barrier, preventing the diffusion of oxygen generated during electrolyte decomposition and lithium-ion insertion reactions. This effectively prevents the formation of an oxide layer on the surface of the metal current collector, thereby preventing degradation;
(2) The conductive layer with a well-designed formulation exhibits good conductivity, enabling the formation of large contact areas with low interface resistance between the current collector and the active coating. This facilitates rapid charge transfer processes;
(3) The flexibility and mechanical buffering of the conductive layer can enhance the adhesion of the physical interface, thereby minimizing the loss of contact area caused by stress generated at the interface during long-term cycling reactions. Through the design and development of unique conductive coatings, experimental results have demonstrated that the conductive interface layer can significantly improve electrochemical performance, such as reversible capacity, capacity retention, and rate capability.
4.References
[1] Busson, C, Blin, M.A., Guichard, P., Soudan, P., Crosnier, O., Guyomard, D., & Lestriez, B. (2018). A primed current collector for high performance carbon-coated LiFePO4 electrodes with no carbon additive. Journal of Power Sources, 406, 7-17.
[2] 孔令涌,黄少真,尚伟丽,等. LiFePO4/膨胀石墨复合材料的制备及性能[J]. 电子元件与材料,2016,35(1):64-67.
[3] 邓龙征,吴锋,高旭光,等 .涂碳铝箔对磷酸铁锂电池性能影响研究[J]. 无机化学学报,2014(4):770-778.
[4] 马守龙,杨茂萍,刘兴亮,等 .铝箔涂碳对LiFePO4/C电池性能的影响[J]. 电池,2017,47(1):39-42.
[5] 杨泛明,焦奇方,伍伟,等 . 涂碳铝箔对LiFePO4动力电池性能影响[J]. 化工进展,2019,38(10):4639-4644.

