A Systematic Solution for Quick Test Diaphragm Ionic Conductivity
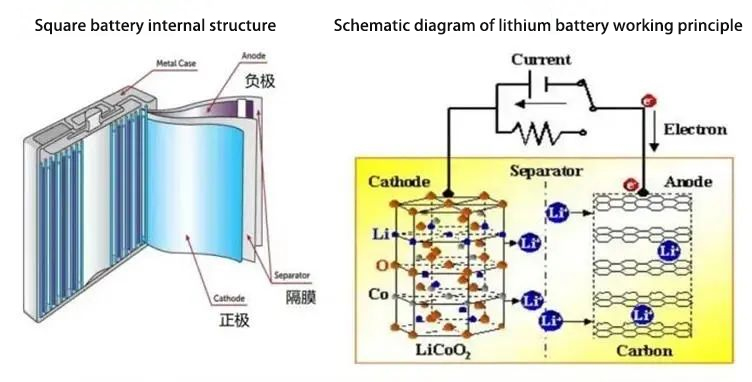
Cathode, anode, electrolyte and separator are the four main materials that make up lithium-ion batteries. During the charging process of a lithium-ion battery, lithium ions are detached from the cathode transported through the separator through the electrolyte, and then embedded in the anode, the discharge process is the opposite. Because the separator plays the role of isolating cathode and anode in the battery, the separator must meet the following points:
1.Electronic insulation;
2.It is easily infiltrated by electrolyte and has a certain ability to retain liquid;
3.Good mechanical properties and structural stability;
4.It has good chemical stability against electrolytes, impurities, and cathode reactants;
5.It can effectively prevent the migration of particles, colloids or soluble substances between the two poles;
6.It has a certain porosity, which enables rapid transmission of ions.
In liquid batteries, the existing separator materials are usually PE material, PP material or a mixture of the two (PP, PE, PP/PE/PP). this type of material undergoes certain processes and is stretched to obtain a membrane base material of a certain thickness. This type of membrane must have a high porosity to ensure ion transmission performance. However, because the battery generates a lot of heat during charging and discharging, PP and PE separators will thermally shrink at high temperatures. To improve the heat shrinkage performance of the separator, the separator is usually coated with nano-alumina or boehmite powder [1].
The ion transport performance of the separator is mainly determined by the porosity and tortuosity of the separator. For the detection of ion transmission performance of separators, the ion conductivity test method in 6.6.2 of《GB/T 36363-2018: Polyolefin Separators for Lithium-Ion Batteries》is currently commonly used in the industry [2].
Figure 1. Schematic diagram of the internal structure and working principle of the battery
Experimental Part
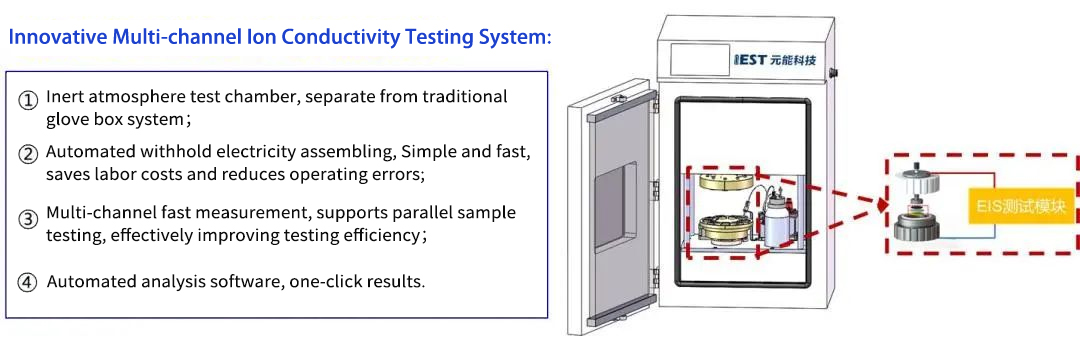
Test Equipment: IEST independently developed multi-channel ion conductivity test system is used for testing. As shown in Figure 2. The instrument includes a four-channel symmetrical battery assembly jig, an electrochemical impedance test system, fitting software, etc. It can be combined with high-purity argon or nitrogen to control the inert atmosphere of the test environment to achieve electrochemical impedance spectroscopy testing of multi-channel symmetrical batteries or different separators.
Figure 2.IEST multi-channel ion conductivity test system
Test Steps: To verify the consistency of the equipment and the actual test conditions, we used separators treated with different processes and different aging temperatures to conduct ionic conductivity tests. The main experimental procedures are as follows:
1.Cut the diaphragm into small diaphragms of a certain area, and bake them in vacuum at 60°C for 12 hours.
2.After baking, the diaphragm is assembled in the mold and transferred to the instrument.
3.Perform vacuuming-inflating and other steps to remove the relevant air and moisture in the sample.
4.Automatically and quantitatively inject liquid (300μL, 1M LiPF6, EMC:EC: DMC=1:1:1) into each channel, and let it sit for a certain period after completing the injection.
5.After the rest is completed, the EIS module of the IEST multi-channel ion conductivity testing system is used to conduct impedance testing.
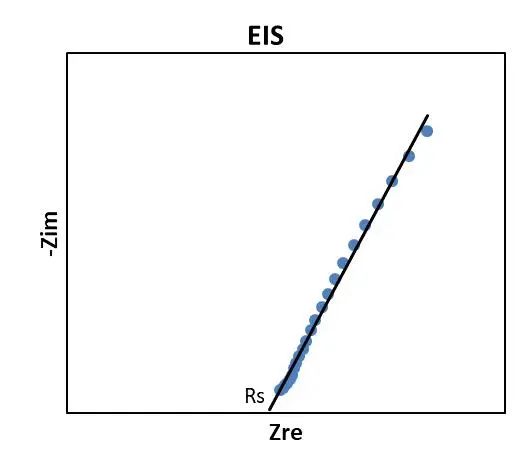
6.Complete the assembly and testing of 1 to 4 layers of diaphragms respectively, use automatic fitting software, and use the obtained EIS as the baseline for fitting. The intersection point of the impedance and the X-axis is Rs, as shown in Figure 3. By fitting the EIS test results of the n-layer diaphragm, the resistance Rs(n) of the n-layer diaphragm can be obtained.
Figure 3.Diaphragm fitting diagram of separator ion conductivity Rs
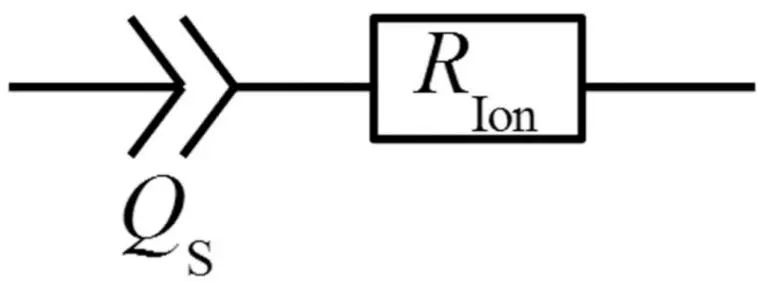
The separator is a porous structure with electrolyte filled in the pores. The equivalent circuit of its AC impedance spectrum can be described by a series circuit of a CPE phase element and an ion resistor, as shown in Figure 4. The impedance of its equivalent circuit is given by the following equation.
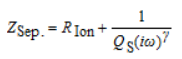
Among them, in the corresponding Nyquist plot (Fig. 3), we simply determine the ionic resistance R Ion within the porous separator by high-frequency extrapolation (ω → ∞), that is, the intersection of the impedance spectrum with the real axis Rs.
Figure 4. Equivalent circuit of the electronically insulating porous separator under blocking conditions
Result Analysis
Case 1: Different Separator Treatment Processes
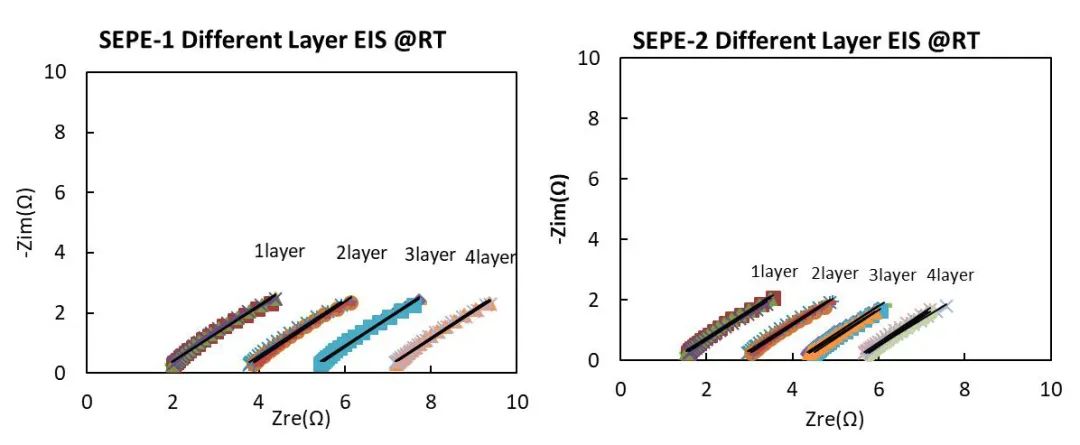
Two separators with the same thickness but different processing techniques are used. The total thickness is 12 μm, named SEPE-1 and SEPE-2 respectively. The test EIS is shown in Figure 5. We can find that there is a big difference between the two EISs, where SEPE-1>SEPE-2.
Figure 5. Diaphragm EIS after different processes
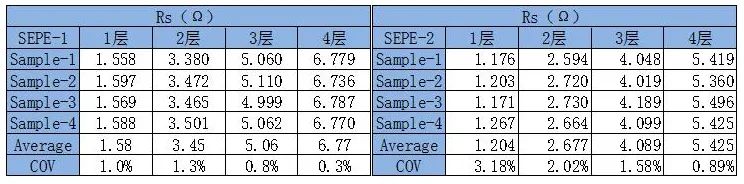
Through fitting calculations, we can obtain the Rs values of SEPE-1 and SEPE-2 under different numbers of layers. The COV of Rs values are all within 5%, indicating good consistency among different parallel samples and stable and reliable test results.
Table 1. Resistance test fitting results for different layer numbers
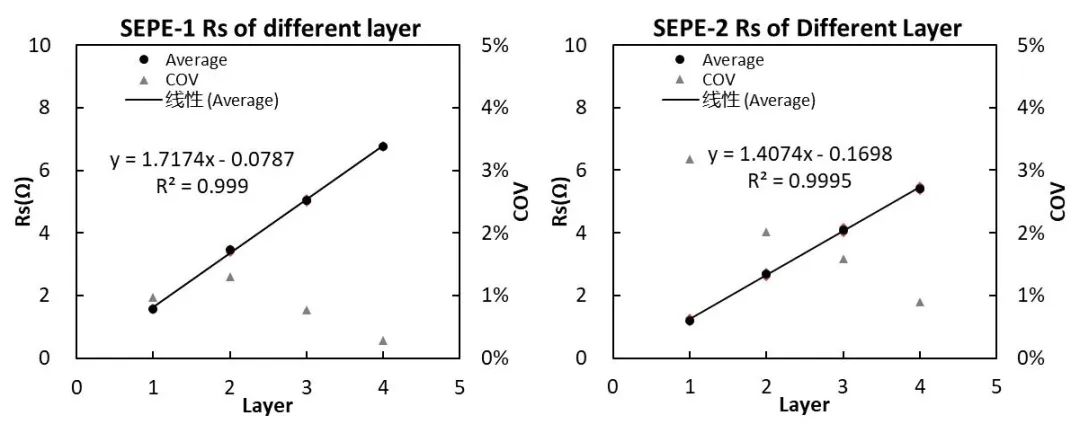
Using the number of layers as the X-axis and Rs as the Y-axis, the relationship between the number of layers and resistance Rs can be obtained, as shown in Figure 6.
Figure 6.Separator Rs after treatment with different processes
According to the national standard formula, the ionic conductivity of SEPE-1 can be calculated to be 0.454mS/cm, and the ionic conductivity of SEPE-2 can be calculated to be 0.554mS/cm, the ionic conductivity of separator SEPE-1 is smaller than that of separator SEPE-2, indicating that SEPE-2 has a better treatment process. The ion conductivity of the membrane depends on many factors, including the material, thickness, pore size, porosity, etc. of the membrane.The ceramic coating structure on the surface of the diaphragm also has an important impact on the ion conductivity. Whether it is the surface coating layer or the diaphragm body, a diaphragm with a larger pore size can increase the ion transmission rate, a separator with higher porosity can increase the number of ion channels, thereby increasing the ionic conductivity. Different processing techniques lead to different separator microstructures and therefore separators with different ionic conductivities.
Case 2: Aging temperatures of different battery cores
After the lithium-ion battery is formed, it is usually left at a certain temperature for a period of time, so that the battery polarization is fully released, the side reactions of the battery cell are also more complete, and the battery cell interface is more stable. This process is called aging in the battery technology. During the aging process, different ambient temperatures and standing times have a great impact on the performance of the battery cells. In order to explore the effect of aging temperature on the separator, we left the formed battery core at room temperature and high temperature of 60°C for 48 hours. Then the battery core was disassembled and the separator was taken out for ionic conductivity testing.
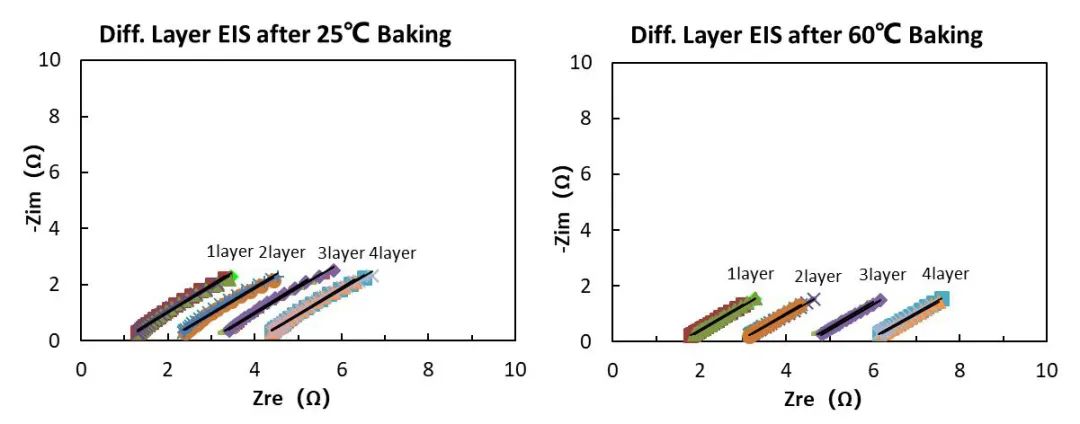
The separator material is PP, the base material thickness is 9μm, and the double-sided ceramic coating process is used in the process, and the single-sided coating thickness is 2μm, that is, the 9+2+2 process. The EIS test result is shown in Figure 7. We can find that the EIS of the separator aged at 60°C is greater than the separator aged at 25°C.
Figure 7. EIS of separators after different aging temperatures
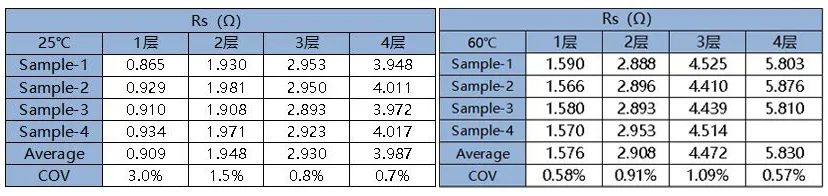
Through fitting calculations, we can obtain the Rs values of the separator under different number of layers at two aging temperatures. The data shows that the Rs value and its COV are all within 5%, indicating good consistency between different parallel samples and stable and reliable test results.
Table 2. Resistance test fitting results after treatment with different aging temperatures
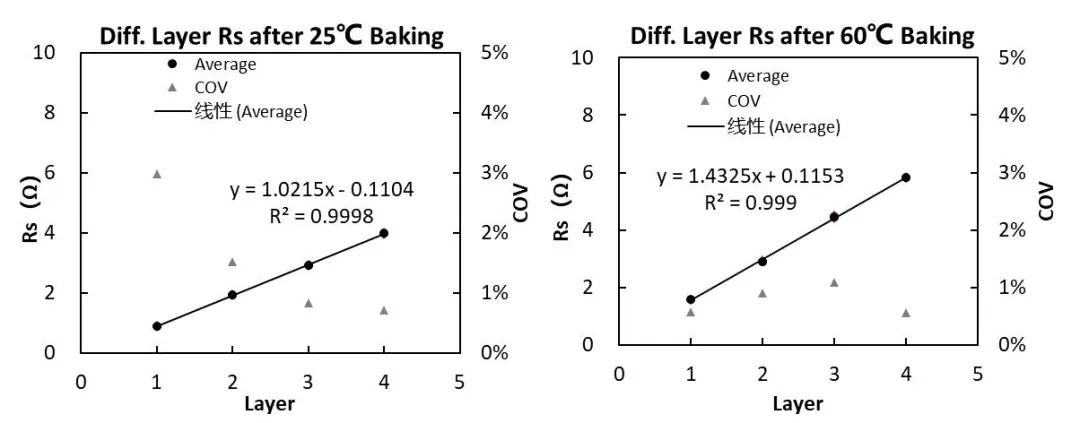
Using the number of layers as the X-axis and Rs as the Y-axis, the relationship between the number of layers and resistance Rs can be obtained, as shown in Figure 8.
Figure 8. Separator Rs after different aging temperature treatments
According to the national standard formula, it can be calculated that the ionic conductivity of the separator after aging temperature treatment at 25°C is 0.803mS/cm, and the ionic conductivity of the separator after aging temperature treatment at 60°C is 0.509mS/cm. It shows that the ion conductivity of the separator after aging treatment at 25°C is higher than that of the separator aged at 60°C.
The main reason is that aging caused by high-temperature storage will cause deposits to appear on the surface of the positive electrode material particles of the battery, which is mainly due to the oxidation of the electrolyte on the surface of the positive electrode particles during the high-temperature storage process. At the same time, sediments may also appear on the surface of the negative electrode, mainly due to the thickening reaction of the SEI film on the negative electrode surface and the decomposition reaction with the electrolyte. These deposits may cover the surface of the membrane and cause pore clogging. At room temperature, the degree of these side reactions is lower and the rate is slower. Therefore, high-temperature aging is more likely to cause the ionic conductivity of the separator to decrease. Therefore, we can determine that the aging process for 48 hours at 60°C may not be suitable for this type of battery.
Summarize
For separators treated with different processes and separators at different aging temperatures, IEST uses self-developed equipment: a multi-channel ion conductivity measurement system to quickly complete the assembly of multi-layer separators and conduct ionic conductivity tests, greatly improving the testing efficiency. At the same time, the test results also show that different membrane treatment processes and different aging temperatures have a greater impact on the ionic conductivity of the membrane.
Reference Literature
[1] Yang Baoquan, Ceramic coating separator modified lithium-ion battery separator, Aging and Application of Synthetic Materials, 2018, 47(1), 67-72.
[2] National standard: GB/T 36363-2018《Polyolefin separator for lithium-ion batterie》.
[3] Wall,Wolfgang,A,et al.Tortuosity Determination of Battery Electrodes and Separators by Impedance Spectroscopy (vol 163, pg A1373, 2016)[J].Journal of the Electrochemical Society, 2017, 164(4).