Limiting Factors of Fast Charging Technology and Analysis of Lithium Stripping Windows
1.Background
With the rapid development of new energy vehicles, consumers have increasingly higher demands for the charging time and driving range of electric vehicles (EVs). Battery fast-charging technology has become a core competitive advantage for power battery companies participating in future market competition and is undergoing rapid iterative innovation. Clearly identifying the factors limiting fast charging is beneficial for promoting innovation and development in battery technology. It can provide guidance and insights for the development of new materials, exploration of new processes, and optimization of battery design and systems. This, in turn, drives progress and innovation in lithium-ion battery fast-charging technology.
2. Limiting Factors
The main factors that limit the fast-charging performance of lithium-ion batteries are as follows:
(1) Material Factors: When a lithium-ion battery is charging, Li+ is transferred from the positive electrode to the negative electrode through the electrolyte. In this process, the transmission path of Li+ mainly includes: 1) the diffusion of Li+ in the cathode material; 2) the diffusion of Li+ through the cathode/electrolyte interface (CEI); 3) Li+ is solvated by solvent molecules in CEI; 4) Diffusion and migration of solvated Li+ in the electrolyte; 5) Desolvation of Li+ at the anode/electrolyte interface (SEI); 6) Li+ enters the anode through SEI; 7) Diffusion of Li+ in the anode [1-2].Therefore, positive and negative electrode materials, electrolytes, separators, battery structures, etc. will all affect the fast charging performance of the battery.
(2) Environmental Factors: First, environmental temperature has a significant impact on the fast charging performance of lithium-ion batteries. In a low-temperature environment, the viscosity of the electrolyte of lithium-ion batteries increases, the ion conduction speed slows down, and the electrochemical reaction rate inside the battery decreases, resulting in an increase in the internal resistance of the battery during the charging process, thus affecting the fast-charging performance [3]. In addition, the diffusion rate of Li+ decreases in low temperature environments, and the charging speed becomes slower. At the same time, the available capacity of the battery will also be reduced in low-temperature environments, affecting battery life. In high-temperature environments, the internal chemical reactions of lithium-ion batteries accelerate, which helps to increase the charging speed. However, the heat dissipation problem of batteries becomes prominent in high-temperature environments. If the heat is not controlled properly, it may lead to safety issues such as battery overheating and expansion. In extreme cases, when the battery temperature exceeds the safety threshold, thermal runaway may occur. Therefore, in order to achieve fast charging of lithium-ion batteries, they need to be charged within an appropriate temperature range. Some electric vehicle battery packs are equipped with heating or cooling systems to ensure that the batteries work within the optimal temperature range. At the same time, charging equipment should also have over-temperature protection functions to prevent the battery from overheating. Secondly, the ambient humidity during the battery manufacturing process will also affect the fast-charging performance of lithium-ion batteries. If the ambient humidity is too high, the moisture content inside the battery will be higher, which may cause a short circuit or redox reaction inside the battery, thus affecting the battery's performance and life. Therefore, a dry environment needs to be maintained during battery manufacturing to avoid the effects of high humidity. In addition, the ambient gas composition during battery manufacturing is also a factor to consider. If the concentration of oxygen or carbon dioxide is too high, it may react with the chemicals inside the battery, affecting the performance and life of the battery. When manufacturing batteries, the environment needs to be well ventilated to avoid the influence of high concentrations of harmful gases.
(3) Charging Method: The charging method used (such as constant current charging, constant voltage charging, pulse charging, etc.) will also affect the battery's fast charging performance. First, constant current charging charges the battery by maintaining a constant current intensity. The advantages of constant current charging are simple control and fast high-rate charging. However, because the terminal voltage of the battery gradually increases as charging progresses, constant current charging is not suitable for long-term charging. In addition, constant current fast charging may cause problems such as battery overheating and overcharging, affecting the efficiency and life of the battery. Secondly, constant voltage charging is to charge the battery by maintaining a constant voltage. Generally, constant current charging to the cut-off voltage is often used, and then constant voltage charging is used to eliminate the uneven distribution of lithium-ion concentration in the battery. The advantage of constant voltage charging is that it is suitable for long-term charging, but because the battery current gradually decreases as charging progresses, the charging speed of constant voltage charging is slower. Constant voltage charging will lead to higher material capacity utilization, greater delithiation and lithium insertion, which may lead to more serious conditions such as damage to the internal materials of the battery, affecting the efficiency and life of the battery. Pulse charging achieves rapid charging of the battery through intermittent fast charging and short-term charging pauses. Pulse charging can alleviate the problem of battery polarization and increased internal resistance, thereby improving the battery charging speed and efficiency. Finally, many studies have proposed that multi-stage constant current charging can slow down battery aging and reduce charging time. The goals of these studies are usually to reduce heat production, avoid lithium precipitation, or reduce mechanical stress. Multi-stage constant current charging involves two or more constant current stages followed by a constant voltage stage. Since the negative electrode potential at the beginning of charging is not easy to drop to the lithium evolution potential, the current in the early constant current stage is larger, which helps to increase the charging speed of lithium-ion batteries.
(4) Impact of Lithium Evolution: Lithium evolution refers to the process in which Li+ forms lithium metal on the surface of the negative electrode during fast charging. First of all, lithium deposition will cause the battery capacity to decay. When lithium metal is deposited on the surface of the negative electrode, it will occupy more volume, resulting in changes in the structure of the negative electrode and a reduction in capacity, limiting the energy density and charging capacity of the battery, and reducing the charging rate. Secondly, the deposition of lithium metal will lead to the continuous formation of SEI film, which will increase the internal resistance of the battery. In addition, lithium dendrite growth is considered a poor interface side reaction[4]. If lithium dendrites penetrate the separator and reach the positive electrode, the battery will undergo an internal short circuit, causing the battery to rapidly generate heat. If heat is not properly controlled, it may lead to battery overheating, expansion, and thermal runaway problems, further limiting the application of fast charging. In conclusion, lithium precipitation is an important factor affecting the fast-charging performance of lithium-ion batteries.
(5) Manufacturing Technology: The manufacturing technology not only determines the structure and performance of the battery, but also affects the safety and life of the battery. First, the preparation process of the electrode has an important impact on fast charging performance. The particle size, distribution and porosity of the electrode all affect the transmission speed and insertion/extraction efficiency of lithium ions. Smaller electrode particles can provide a larger specific surface area, shorten the transmission path of lithium ions, increase the embedding/extraction speed, and help improve fast charging performance. Secondly, the thickness and porosity of the separator also affect the fast-charging performance. A thin separator and appropriate porosity can reduce internal resistance, improve lithium-ion transmission efficiency, and facilitate fast charging. At the same time, the strength and thermal stability of the separator must also be guaranteed to ensure battery safety and stability. Finally, the assembly process of the battery will also affect the fast-charging performance. Reasonable battery structural design, assembly process and assembly method of each component will affect the overall performance and safety of the battery. For example, whether the conductive agent inside the battery is evenly distributed, the contact between the current collector and the active material, etc. will affect the transmission efficiency of electrons and ions, thereby affecting the fast-charging performance. To improve fast charging performance, it is necessary to optimize key aspects such as electrode preparation, separator selection, battery structure and assembly process. This requires advanced manufacturing equipment and fine process control to ensure battery consistency and reliability.
(6) Safety Factors: Fast charging technology needs to consider the safety of the battery, such as avoiding overheating, overcharging, over-discharging, etc. These factors may also affect the fast-charging performance of the battery.
In general, there are many factors that affect the fast-charging performance of lithium-ion batteries, including materials, charging environment, charging methods, lithium deposition, manufacturing processes and safety factors. In order to improve the fast-charging capability of lithium-ion batteries, it is necessary to optimize the charging strategy and control the changes in charging current and voltage so that they fluctuate reasonably within the lithium evolution window.
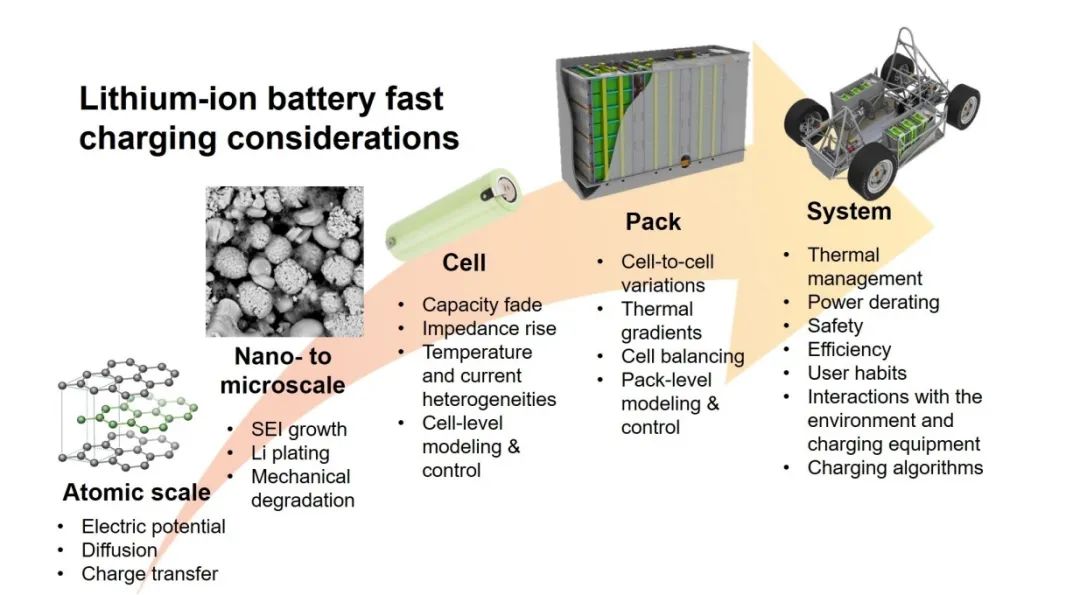
Figure 1. Factors affecting the fast charging of lithium-ion batteries at different levels[5]
3.Lithium Stripping Window Testing Method
The traditional method is to dismantle the fully charged core and observe whether there is gray-white lithium deposits on the surface of the negative electrode. However, the disassembly method is an out-of-situ method. On the one hand, it causes irreversible damage to the battery core. On the other hand, it can only determine the approximate lithium evolution rate or temperature and cannot obtain an accurate lithium evolution window. In addition, in-situ optical imaging technology can also be used to characterize lithium evolution and can directly conduct dynamic observation and analysis of the lithium-ion deposition process and growth morphology. However, it has the disadvantages of special structural requirements for the battery, poor resolution, and inability to perform quantitative analysis.
Three electrodes detect the negative electrode potential: when the negative electrode potential is lower than a certain potential such as 0V (vs Li+/Li), lithium deposition occurs on the negative electrode. By detecting the negative electrode potential through the reference electrode in the battery, the impact of different working conditions on the lithium evolution of the negative electrode can be obtained. However, when assembling the three electrodes, attention should be paid to the selection and placement of the reference electrode, both of which will directly affect the accuracy and reproducibility of the negative electrode potential acquisition. To solve the limitations of traditional methods, IEST uses the self-developed in-situ expansion analyzer (SWE) to quantitatively evaluate the expansion thickness of the battery core at different charging rates, which can determine the lithium precipitation voltage and SOC window of the battery core at different charging rates, which provides a new method for R&D personnel to formulate fast charging strategies.
Figure 2. Schematic diagram of the in-situ expansion analyzer
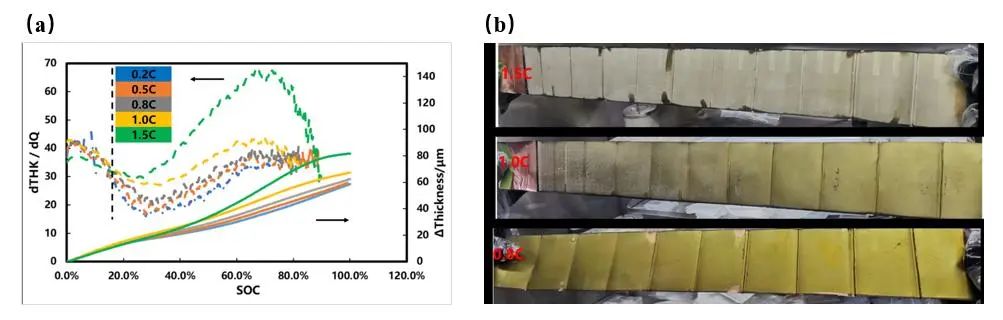
Figure 3. (a) Expansion thickness curves at different charging rates and (b) disassembled pole piece after full charging
Different charging rates are used to charge the battery. It can be clearly seen from Figure 3(a) that the 1C and 1.5C rate expansion thickness curves when charged to about 15% SOC, with the other three rate expansion thickness curves, a "bifurcation" phenomenon began to occur. It is speculated that as the charging rate increases, the battery polarization increases, and lithium precipitation occurs on the surface of the negative electrode, which leads to an acceleration of the battery cell expansion rate. To verify whether there is lithium precipitation in the battery core, the fully charged battery core was disassembled to observe the negative electrode surface, as shown in Figure 3(b), when fully charged at a rate of 1.5C, the entire surface of the negative electrode plate appears gray-white. When fully charged at a rate of 1C, part of the surface of the negative electrode plate appears gray-white, indicating that both have different degrees of lithium precipitation, the negative electrode piece that is fully charged below 0.8C rate appears golden yellow, and no lithium precipitation is observed.
The charging rate determines the lithium-ion flux on the negative electrode material per unit area. When the solid phase diffusion process of Li+ in the negative electrode is slow (for example, when the temperature is too low, the state of charge is high or the diffusion of Li+ in the material needs to overcome a large activation energy), when the charging current density is too high, lithium ions will continue to accumulate on the surface of the negative electrode, and the potential of the negative electrode will continue to become negative. After electrons are obtained, a lithium evolution reaction occurs. When lithium is embedded in graphite, the volume of the material itself expands by about 10%. Considering that the pole piece itself has a porous structure, the actual expansion rate of the pole piece thickness is even lower. When a loose lithium deposition layer is precipitated on the surface of the graphite pole piece, the thickness change rate of the pole piece is greater, according to the above expansion curve and the experimental results of disassembling the lithium state, by comparing the battery expansion curves at different rates, the charging rate and SOC range of the lithium can be quickly determined.
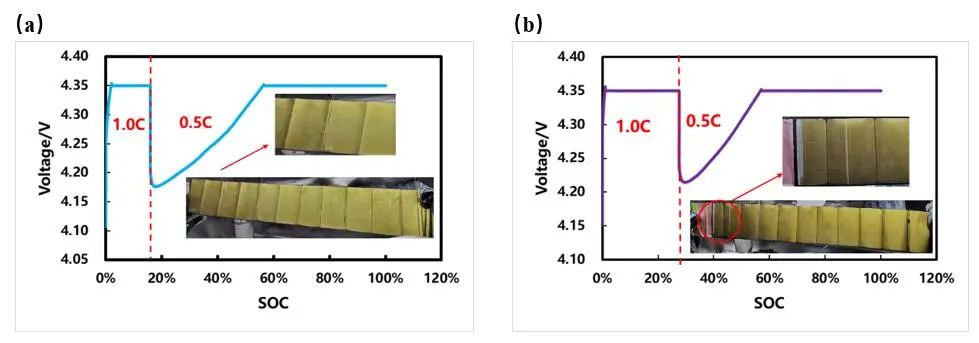
Figure 4. (a) Unprecipitated lithium and precipitated lithium during step rate charging (b) Battery cell charging curve and disassembly picture
To further verify the SOC at which lithium evolution begins, we conducted two sets of step charging experiments at different rates. before and after the inflection point of the thickness expansion curve appears, charging is switched to a smaller rate: a group of cells is charged at a constant current of 1C to about 15.7% SOC and then charged at 0.5C to full power (as shown in Figure 4(a)), the other set of cells is charged at 1C constant current to approximately 27.4% SOC and then charged at 0.5C to full power (Figure 4(b)). After dismantling the battery core, it was found that slight lithium precipitation occurred on the surface of the negative electrode after changing the rate to 27.4% SOC, and there was no lithium precipitation on the surface of the negative electrode after charging to 15.7% SOC. This shows that the lithium evolution SOC during 1C rate charging occurs between 15.7% and 27.4%. Compared with Figure 3(a), it is basically consistent with the SOC position corresponding to when the slope of the 1C expansion thickness curve begins to bifurcate. The solid-phase diffusion coefficient of lithium ions in graphite particles is related to the charge state of the material. As the SOC changes, when the solid-phase diffusion coefficient is relatively low, the lithium precipitation phenomenon is more likely to occur. This non-destructive lithium-elimination detection method can quickly determine the lithium-elimination SOC window during battery charging, providing effective guidance for formulating fast charging strategies.
4.Reference Materials
1.Qixin Gao, Jingteng Zhao, Guoxing Li. Research progress on fast-charging lithium-ion batteries. Energy Storage Science and Technology, 2023, 12: 2166-2184.
2.Guoxing Li. Regulating mass transport behavior for high-performance lithium metal batteries and fast-charging lithium-ion batteries. Advanced Energy Materials, 2021, 11:202002891.
3.Chandrasekaran R. Quantification of bottlenecks to fast charging of lithium-ion-insertion cells for electric vehicles. Journal of Power Sources, 2014, 271: 622-632.
4.Waldmann T, Hogg B I, Wohlfahrt-Mehrens M. Li plating as unwanted side reaction in commercial Li-ion cells-A review. Journal of Power Sources, 2018, 384: 107-124.
5.Anna Tomaszewska, Zhengyu Chu, Xuning Feng, Simon O’Kane, Xinhua Liu, Jingyi Chen, Chenzhen Ji, Elizabeth Endler, Ruihe Li, Lishuo Liu, Yalun Li, Siqi Zheng, Sebastian Vetterlein, Ming Gao, Jiuyu Du, Michael Parkes, Minggao Ouyang, Monica Marinescu, Gregory Offer, Billy Wu. Lithium-Ion Battery Fast Charging: A Review. eTransportation. 2019, 1: 100011.