Current Research Status of High-Nickel Ternary Cathode Materials for Lithium-ion Batteries
Based on the advantages of high energy density, large discharge capacity, and low overall cost, ternary cathode materials, especially high-nickel ternary materials (NCM), are expected to be the main development trend for future ternary cathodes. This article provides a comprehensive review and discussion of the basic characteristics, inherent challenges, and current research status of high-nickel ternary materials.
1.The Fundamental Characteristics of High-Nickel Materials
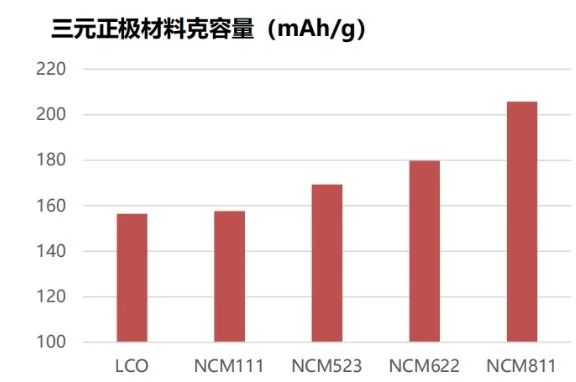
Due to the inherent characteristics of the elements, different elements play different roles in the structure and electrochemical performance of NCM ternary materials. Generally, in the NCM system, the higher the nickel content, the higher the gravimetric capacity (as shown in Figure 1); the higher the cobalt content, the better the rate performance; and the higher the manganese content, the more stable the structure. Therefore, the performance of ternary materials changes with the alteration of element ratios. According to the nickel content, NCM can be mainly classified into NCM111, 532, 622, 811, etc., and transitional products such as 721, among which 622 and above are considered high-nickel ternary materials.
Figure 1. Gravimetric Capacity of Different Ternary Cathode Materials
2. Lssues With High-Nickel Materials
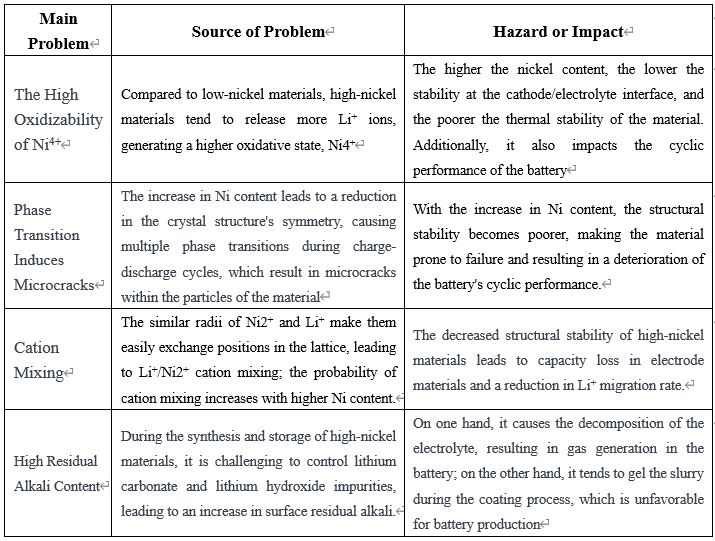
High-nickel ternary materials, ranging from NCM532 and NCM622 to NCM811, as well as NCA ternary materials, have been iteratively updated. Increasing the Ni content to 90% or above not only helps reduce costs but also significantly enhances the battery's specific energy. However, as the Ni content increases, high-nickel ternary materials also face numerous issues and challenges in practical applications. Table 1 summarizes some of the inherent problems associated with high-nickel ternary materials.
Table 1: Some Inherent Issues of High-Nickel Ternary Materials:
3. Research on the Modification of High-Nickel Materials
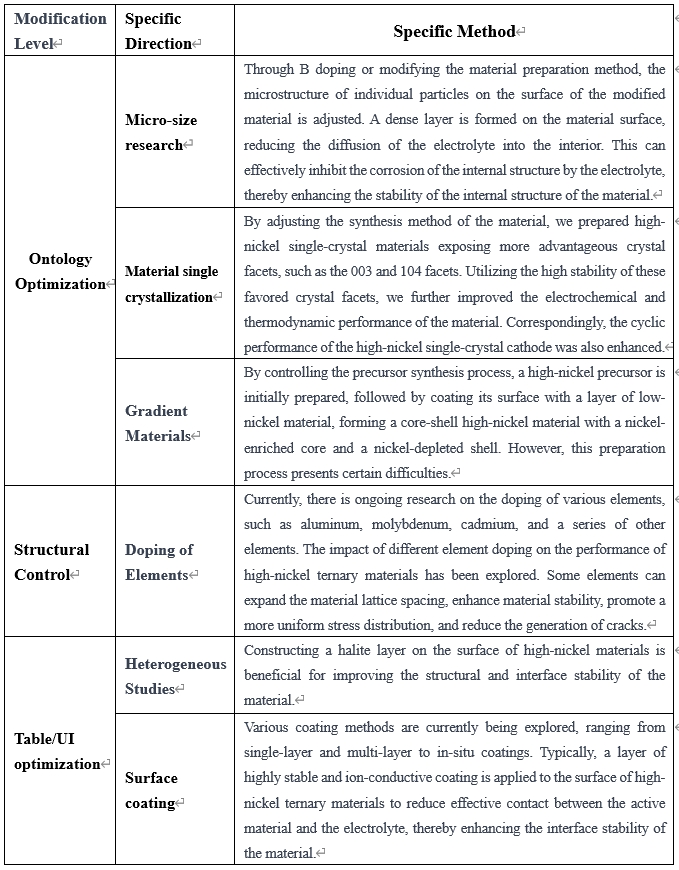
To overcome the aforementioned issues in the application of high-nickel ternary materials, it is often necessary to carry out material-level modifications to enhance the electrochemical and safety performance of subsequent batteries. Currently, research on the modification of high-nickel ternary materials can be categorized into several directions: material intrinsic optimization, material structure regulation, and material surface/interface enhancement, as shown in Table 2:
Table 2: Modification Approaches for High-Nickel Ternary Materials Reported to Date:
4.Summary of High-Nickel Materials Research
(1)High-nickel ternary cathode materials continue to iteratively develop, and nickel enrichment can enhance the energy density of batteries. However, inherent issues in high-nickel ternary materials need targeted improvement and optimization.
(2)Through methods such as intrinsic material optimization, element doping, and surface coating, the defects in high-nickel ternary cathode materials can be effectively addressed, improving and enhancing their performance in battery applications.
(3)High-nickel ternary materials remain a research focus for high-energy cathodes. If safety and cycle life issues of high-nickel ternary materials are resolved, there is significant potential for their industrial applications.
5.High-Nickel Material Testing Cases
(1) Conductivity and Compressive Performance Evaluation of Different NCM Material Systems.
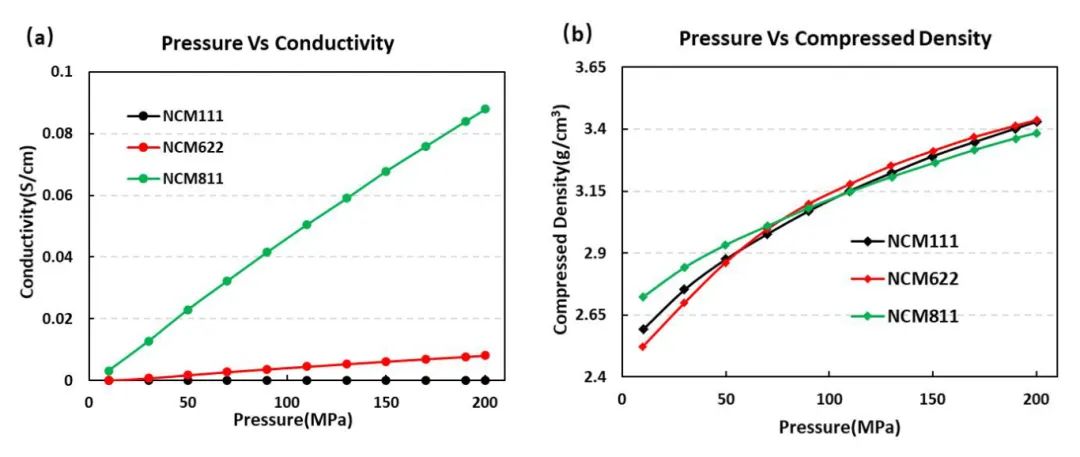
This study employed the Powder Resistivity & Compaction Density Tester PRCD3100 (IEST) to assess the electrical conductivity and compressive performance of NCM111, NCM622, and NCM811 materials (Figure 3). It was observed that the conductivity gradually increased with an increase in nickel content. However, under the current compression conditions, the compressive modulus of NCM622 was greater than that of NCM111 and NCM811. This is attributed to its microstructure, as evident from the SEM morphology analysis of the three materials. NCM622, composed of a layered structure of polycrystalline material, is more easily compressed, consistent with the actual compression performance experimental results.
Figure 3. Conductivity curves (a) and Compaction Density curves (b) for the three NCM materials
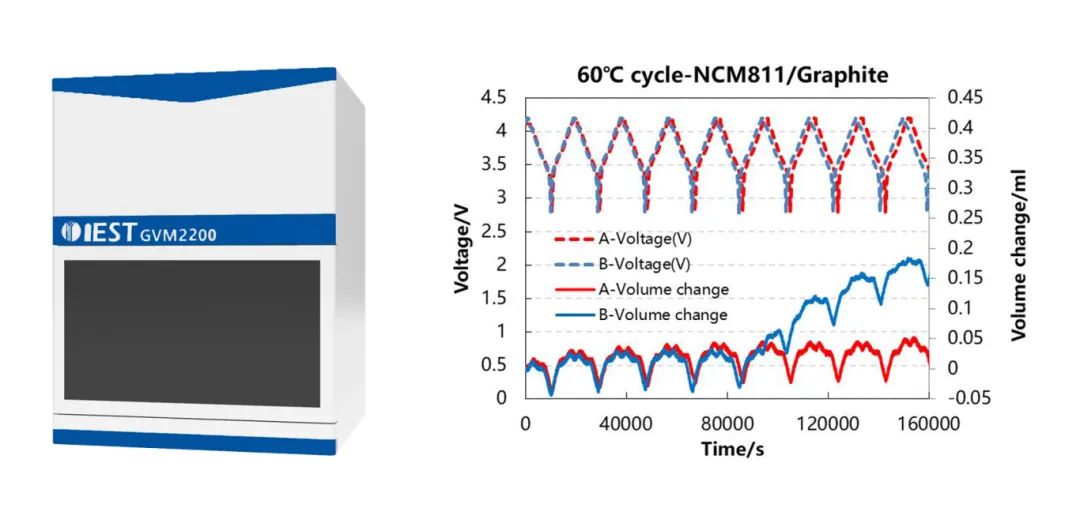
(2) In-situ Analysis of Volume Changes during High-Temperature Cycling of NCM811 Battery Cells.
This study employed the controllable temperature dual-channel in-situ volume monitoring device GVM2200 to analyze the volume changes of NCM811/Graphite battery cells in two different electrolyte systems during high-temperature cycling (Figure 4). This method allows for a visual assessment of the differences in gas evolution between the two electrolyte systems. Additionally, it reveals the volume changes corresponding to the material phase transitions during the lithium extraction and insertion processes, aiding research and development personnel in conducting in-depth mechanistic analyses of material and electrolyte performance.
Figure 4.External view of the GVM2200 device, charge-discharge curves, and volume change curves for NMC811-Graphite battery cells
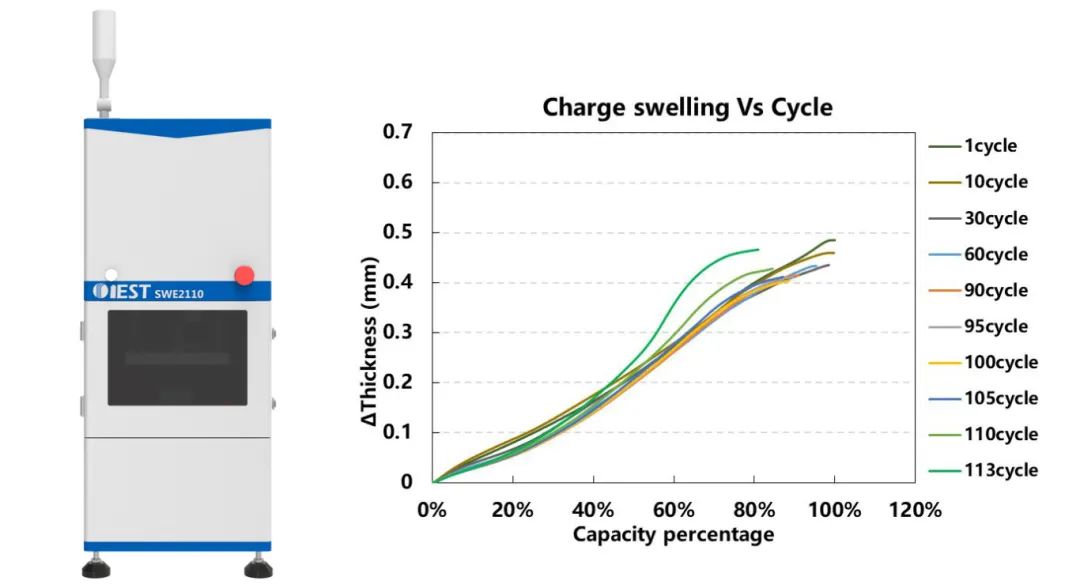
(3) Analysis of Expansion Changes during Long Cycling of Ternary Pouch Cells.
This study employed the in-situ swelling analyzer SWE2110 to analyze the correlation between capacity decay and thickness expansion during the extended cycling of NCM battery cells (Figure 5). Through the analysis of the expansion thickness of the battery cells and electrochemical data, it is speculated that the causes of the cycling degradation in this cell include electrode mechanical damage, lithium plating, and other side reactions.
Figure 5.Schematic diagram of the in-situ expansion analyzer equipment, and variation curves of expansion force during different battery cell cycling and charging.
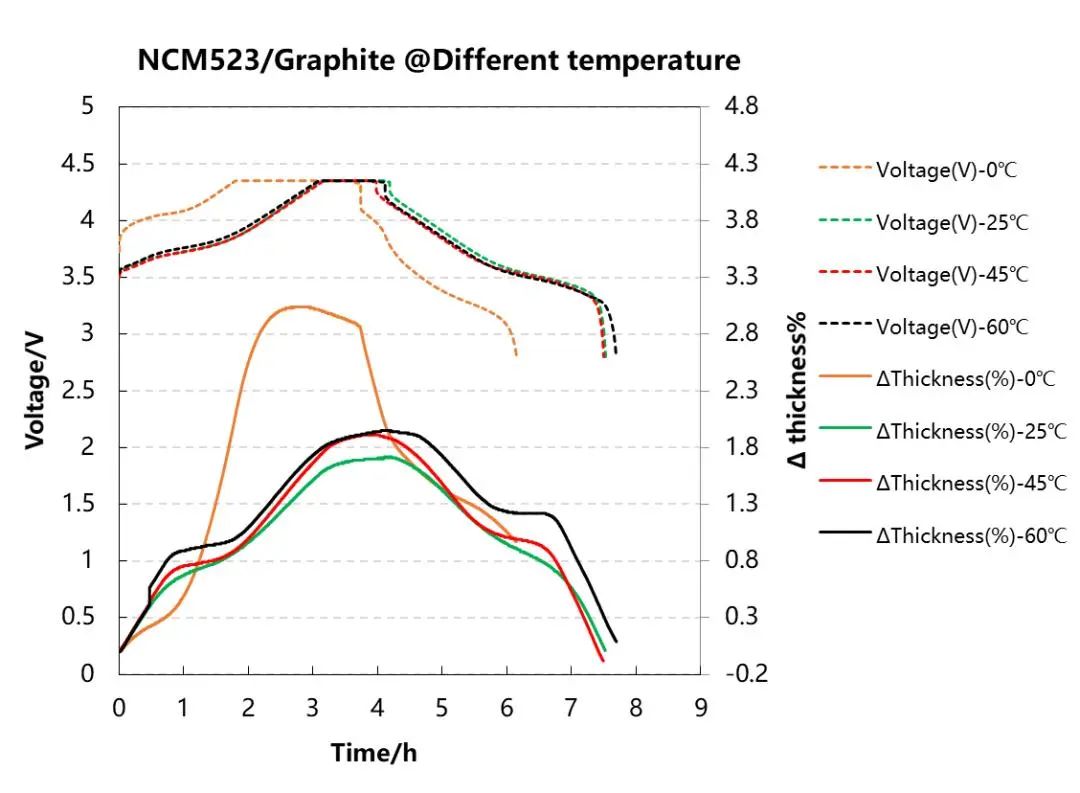
(4) In-situ Expansion Analysis of Ternary Battery Cells under Different Temperature Conditions.
This study utilized the in-situ expansion analyzer SWE2110 (IEST) to analyze the thickness expansion of NCM523 battery cells during charge-discharge processes under different temperature conditions. As the temperature increased from room temperature (25°C) to 45°C and 60°C, as well as decreased from room temperature (25°C) to 0°C, the irreversible expansion of the battery cells increased. However, the reasons for irreversible expansion under high and low-temperature conditions may differ.
Figure 6.Charge-discharge curves and expansion curves of NCM523 battery cells under four different temperature conditions