Effects of Lithium-ion Battery Solvents and Lithium Salts on the Electrode Wetting
At present, lithium-ion batteries have been widely developed in the fields of 3C electronic equipment, electric vehicles and energy storage. The market demand for lithium-ion batteries with higher energy density and better performance has grown sharply. In the development of high-energy-density lithium-ion batteries, how to improve the capacity utilization of electrodes and improve the difficulty of infiltration caused by high compaction density are key technical challenges, effectively improving the wettability of the electrolyte has been identified as the key to solving this problem. In the manufacturing of lithium-ion batteries, uneven wetting will lead to uneven current density distribution and unstable electrolyte interface film (SEI) formed; at the same time, incomplete infiltration directly affects the performance of the battery, leading to the occurrence of lithium deposition and triggering a series of safety issues. In short, the raw material electrolyte for lithium-ion batteries is the core part of the research and development of lithium-ion batteries. The consideration and evaluation of the wettability of the electrolyte to the electrode is the key to the development of high-performance lithium-ion batteries.
The electrolyte for lithium-ion batteries usually uses organic electrolyte, which has good stability. The organic electrolyte mainly contains lithium salts, solvents and additives. The concentration and type of each part directly affect the performance of the electrolyte and battery; Figure 1 is an overview of electrolyte-related research. According to statistics, solvents account for 85% of the mass and 30% of the cost in the electrolyte; The electrolyte accounts for 6%-8% of the cost of power batteries (the cost of electrolyte in mainstream NCM523 battery core materials accounts for about 5.6%, and the cost of electrolytes in lithium iron phosphate (LFP) battery materials accounts for about 8.5%); Power batteries account for 40% of new energy vehicle costs. Comprehensive statistics indicate that solvents account for 1.8%-2.4% of the cost of power batteries and 0.72%-0.96% of the cost of new energy vehicles. The electrolyte uses a "mixed solvent system", 95% of which is "carbonate solvents", which are divided into cyclic carbonates and chain carbonates according to their structures,including dimethyl carbonate (DMC), diethyl carbonate (DEC), ethyl methyl carbonate (EMC), ethylene carbonate (EC), propylene carbonate (PC). The main function of the solvent is to dissolve lithium salts and form conductive ions; the lithium salts mainly play the role of providing conductive ions. Lithium hexafluorophosphate (LiPF6) is the most commonly used lithium salt at present. The viscosity of the electrolyte is jointly determined by the lithium salt and the solvent, and the viscosity is directly related to the wettability of the electrolyte.
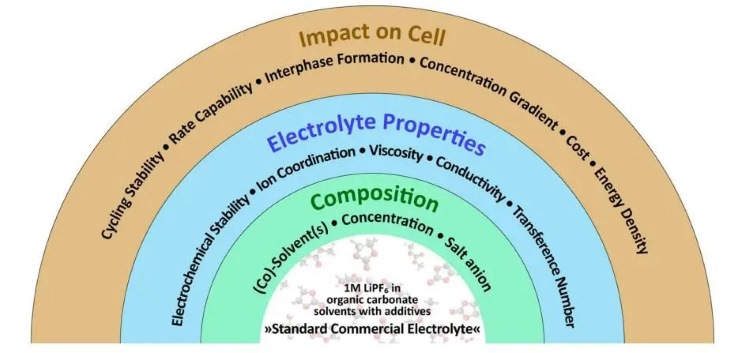
Figure 1.Overview of electrolyte related research
This article mainly combines the Electrolyte Wetting System (EWS1100) developed by IEST, and uses different solvents and electrolytes to evaluate the differences in capillary wettability, provide a feasible test solution for the wettability differences of different solvent and electrolyte systems.
This article is mainly based on the capillary infiltration test system, combined with different compaction densities of negative electrode plates for systematic testing, to evaluate the wettability differences of the electrode plates under different compaction densities.
Experimental Equipment and Testing Methods
1.Experimental Equipment: Model EWS1100 (IEST).
The appearance of the equipment is shown in Figure 2.

Figure 2. Schematic diagram of EWS1000 equipment
2.Sample Preparation and Testing
2.1Sample preparation: Use four solvents of PC\DEC\DMC\EMC and four electrolytes of L01\L02\L03\L04 to conduct wetting testing on the same electrode.
2.2Testing process: Pretreatment of the sample to be tested → Standardized fixation of the sample → Equipment connection and software parameter design → Automatic capillary suction → Automatic capillary pressure test → Real-time monitoring of the capillary liquid level by the visual recognition system → Data collection and processing.
3.Test Principle
Electrolyte Wetting System (EWS1100), It can quantitatively evaluate the difference in infiltration of electrolyte between different positive and negative electrode plates and separators, providing an effective method for electrolyte infiltration evaluation. Figure 3 is a schematic diagram of the test principle of the capillary infiltration method. The capillary glass tube is in contact with the surface of the pole piece, and electrolyte is injected into the capillary. As the electrolyte continues to infiltrate the coating, the capillary liquid level continues to decrease. The visual recognition system records the liquid level height of the capillary in real time. The dynamic evolution process of the liquid level is the real-time process of electrolyte infiltration. The height change is the amount of electrolyte infiltration.
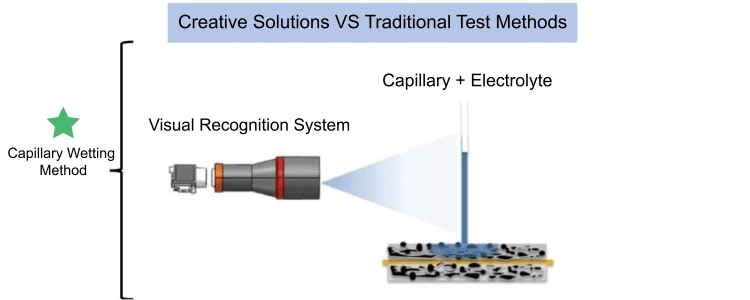
Figure 3. Capillary Wetting Method Test Principle
Wetting Test Results
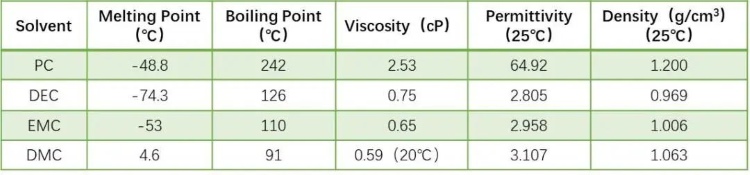
Lithium-ion battery solvents generally use organic solvents with high dielectric constant and small viscosity. The higher the dielectric constant, the easier it is for lithium salts to dissolve and dissociate; the lower the viscosity, the faster the ions move. However, solvents with high dielectric constants usually have high viscosity, and solvents with low viscosity have low dielectric constants. Therefore, in practical applications, multiple solvents are usually mixed to obtain the optimal electrolyte system. Compared with the study of mixed systems, the performance evaluation of a single system is the basis for systematic research and development of electrolytes. In this experiment, four solvents with different properties (for example, Table 1 shows the physical property indicators of the four solvents) were first selected to conduct capillary infiltration characterization at the pole piece level to evaluate the infiltration differences of different solvents under this method.
Table 1. Physical Properties of Various Solvents
Combined with the EWS1100 series electrolyte infiltration equipment, a 10μl capillary tube was used to evaluate the differences between different solvents on the same pole piece, and the data were normalized. For example, Table 2 shows the comparison results of the infiltration amount data of different solvents, and Figure 4 shows the infiltration comparison curves of different solvents. From the slope of the curve, it can be clearly seen that there are obvious differences in the infiltration between different solvents. among them, DMC completed all the infiltration of the sampled liquid in about 20 seconds; judging from the results of the infiltration amount in 10 seconds and 20 seconds, both showed the infiltration trend of PC<DEC<EMC<DMC. This trend is opposite to the result of solvent viscosity. therefore, viscosity is a key indicator in the development and use of commercial electrolyte solutions, in addition, a suitable temperature usually needs to be provided during the cell injection stage. This is also because appropriately increasing the temperature can reduce the viscosity of the electrolyte, thereby accelerating the injection and infiltration process.
Table 2. Differences in Wettability of Different Solvents
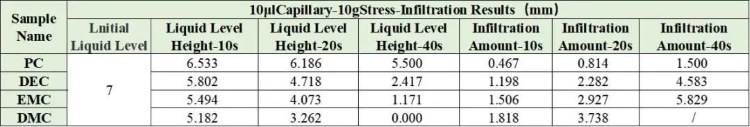
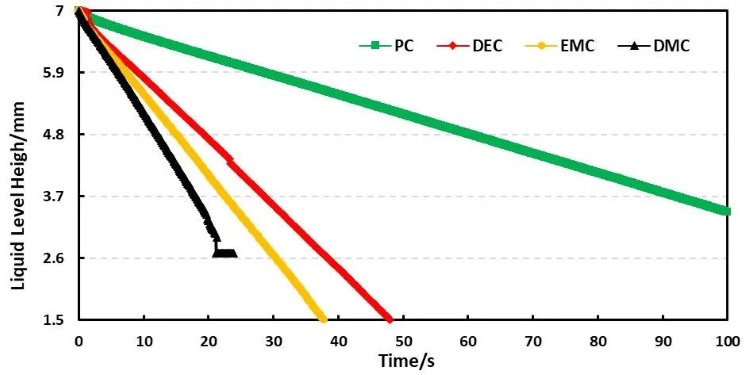
Figure 4. Capillary wetting curves of four different solvents
The electrolyte lithium salt is the basis for the conduction of lithium ions. A suitable lithium salt must have good thermal stability and not easy to decompose, high ion conductivity, good chemical and electrochemical stability, and low cost; Although there are many types of lithium salts, those suitable for lithium-ion batteries are very limited. Currently, lithium salts commonly used in laboratories and industrial production generally choose lithium salts with larger anion radius and stable oxidation and reduction, among them, lithium hexafluorophosphate (LiPF6) is currently the most used lithium salt in lithium-ion batteries. This experiment combines different solvents to carry out electrolyte ratios with a fixed lithium salt concentration, and prepares electrolytes with four ratios as shown in Table 3. The main components are based on PC, EMC, DMC and DEC with the addition of 1M LiPF6 , combined with these four electrolytes, the wettability test under the capillary principle was performed.
Table 3. Ingredients of Four Electrolyte Systems
For example, Table 4 shows the comparison results of the infiltration amount data of four electrolyte systems based on different solvent ratios. Figure 5 shows the comparison curves of capillary infiltration of different electrolytes. From the curve slope, there are obvious differences in the infiltration conditions of the four electrolytes; From the infiltration amount table, the infiltration trend L01<L04<L02<L03 can be further clarified. Combined with the analysis of the electrolyte components, this trend is consistent with the trend of pure solvent without adding lithium salt, but compared with the pure solvent, the wettability of the electrolyte is reduced after adding lithium salt. Comparing DMC with L03 with 1M lithium salt added to it, DMC completed all the initial liquid infiltration in about 20 seconds, while L03 only completed 34.8% of the initial liquid volume in 50 seconds (infiltration volume in 50 seconds/initial liquid level height) ); this result mainly considers that the addition of lithium salt to the solvent increases the viscosity of the liquid, and the change in viscosity directly leads to the decrease in the wettability of the electrolyte.
Table 4. Differences in Wettability of Different Electrolytes
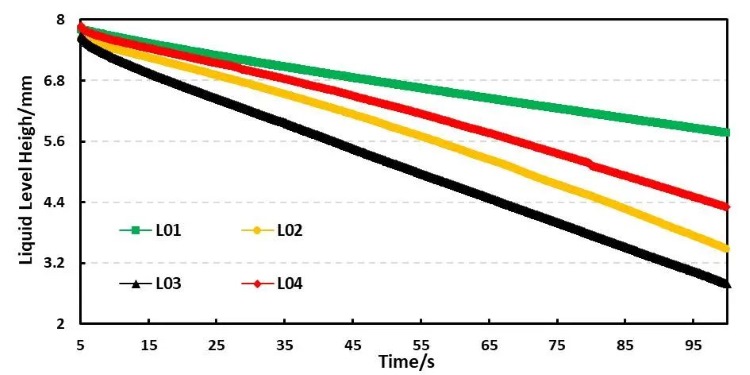
Figure 5. Capillary wetting curves of four different electrolytes
Conclusion
The electrolyte used in lithium-ion batteries is a lithium salt with a suitable concentration dissolved in an organic aprotic mixed solution. A common electrolyte is generally a system composed of a mixed carbonate solvent at a lithium salt concentration of 1M. The selection of lithium salt, solvent and the quality of the electrolyte system determine the cycle efficiency, operating voltage, operating temperature, and service life of the battery, and play a decisive role in the overall performance of the battery. The viscosity of the electrolyte is the key to the wettability. This article evaluates the difference in wettability of solvents with different viscosities and solutions after adding lithium salts, and clarifies the impact of viscosity on wettability. During the development process of the actual electrolyte formula, its infiltration assessment at the pole level can be used as an important reference indicator.
Reference Literature
[1] Sheng Y. Investigation of electrolyte wetting in lithium-ion batteries: Effects of electrode pore structures and solution[J]. Dissertations & Theses – Gradworks, 2015.
[2] Yao N, Yu L, Fu Z H, et al. Probing the origin of viscosity of liquid electrolytes for lithium batteries[J]. Angewandte Chemie International Edition, 2023: e202305331.
[3] Compiled by Zheng Honghe et al. Lithium-ion battery electrolytes. Beijing: Chemical Industry Press, 2007.01
[4] Weydanz W J , Reisenweber H , Gottschalk A ,et al.Visualization of electrolyte filling process and influence of vacuum during filling for hard case prismatic lithium-ion cells by neutron imaging to optimize the production process[J].Journal of Power Sources, 2018, 380(mar.15):126-134.DOI:10.1016/j.jpowsour.2018.01.081.
[5] Wu M S , Liao T L , Wang Y Y ,et al. Assessment of the Wettability of Porous Electrodes for Lithium-Ion Batteries[J]. Journal of Applied Electrochemistry, 2004, 34(8):797-805. DOI:10. 1023/B:JACH.0000035599.56679.15.
