In-situ Quantitative Separation of Soft/Hard Swelling in Lithium-ion Batteries
Preface
With the depletion of traditional energy sources, lithium-ion batteries are widely used in consumer electronics, new energy vehicles, photovoltaic energy storage and other fields due to their long cycle life and high energy density, but the accompanying safety issues have also caused users of great concern.In the early stages of safety failure, lithium-ion batteries often exhibit significant expansion deformation and cause significant pressure changes between cells, and this expansion is much earlier than temperature anomalies and gas overflow phenomena. Therefore, studying the expansion behavior of lithium-ion batteries and summarizing the causes of deformation of lithium-ion batteries is of great significance for improving battery safety and developing a thermal runaway warning system for lithium-ion batteries. On the one hand, the structure of the positive and negative electrode materials undergoes a certain hard expansion due to lithium desorption/intercalation during the charge and discharge process[1-3].On the other hand, lithium-ion batteries will also generate gas due to various chemical or electrochemical reactions under the working conditions of formation, cycle aging, floating charging, and storage [4-6], which will cause the cell to bulge, that is, soft expansion behavior.
Although the manifestations of the two are similar, the formation mechanism is completely different. Lithium-ion batteries will be accompanied by different degrees of gas production and expansion during the entire normal charge and discharge cycle. The electrolyte decomposition is the most important source of gas production. First, because the moisture inside the battery will react with the electrolyte and produce CO₂, H₂, O₂ and other gases; Second, solvents such as EC and DEC in the electrolyte will generate a large amount of free radicals with the side reaction products of the electrode materials, and then release a large amount of hydrocarbon gases through a chain reaction. This paper selects the Si/C anode that has been researched more on the market,using the in-situ volume monitor (GVM) of IEST(Initial Energy Science&Technology) and the in-situ swelling analyzer (SWE),effectively quantitatively separate the soft/hard expansion behavior of NCM/SiC pouch battery during the formation process, which has significant guiding significance for the optimization and improvement of the lithium-ion battery formation process.
1.Experimental Equipment and Test Methods
1.1 Experimental Equipment
Figure 1(a) In-situ gas production volume monitor, model GVM2200; Figure 1(b) in-situ swelling analyzer, model SWE2110.

1.2 Testing Information and Process
1.2.1 The Cell Information is Shown in Table 1
Table 1. Test Cell Information
| Information of Cell | |
Cathode | NCM |
Anode | Si/C |
Capacity | 200mAH |
Size | 60mm*45mm |
1.2.2 Formation and Charging Process
Table 2. Formation Charging Process
No. | Step | Stop Condition | Sampling Frequency |
1 | Rest | 60 min | 30s |
2 | 0.01C CC | Cut-off Voltage 3.0V | 30s |
3 | 0.05C CC | 30 min | 30s |
4 | 0.1C CC | Cut-off Voltage 3.75V | 30s |
1.2.3 Experimental Process
Cell expansion volume test: Put the cell to be tested (with air bag) into the corresponding channel of GVM2200, open the MISS software, set the cell number and sampling frequency corresponding to each channel and other parameters, the software will automatically read the real-time volume, test temperature, Current, voltage, capacity and other data.
Cell expansion thickness test: Put the cell to be tested (with air bag) into the corresponding channel of SWE2110, open the MISS software, set the cell number and sampling frequency and other parameters corresponding to each channel, and the software will automatically read the cell thickness and thickness change Quantity, test temperature, current, voltage, capacity and other data.
2.Result Analysis
2.1 Analysis of the Results of the Total Volume Expansion of the Cell
Place the same batch of cell A in the in-situ gas production volume monitor (GVM2200), set the temperature of the cycle temperature control system at 25°C, and monitor the volume change of cell A in the process of formation in real time, the results are shown in Figure 2 shown. The entire chemical formation process can be divided into four stages: the first is the low-voltage gas production stage at the negative end, mainly producing ethylene (C₂H₄), ethane (C₂H₆) and other gases. The second stage is a stage in which gas generation and consumption coexist, so the slope of the gas production curve is slower than that of the first stage. J.R. Dahn et al. [4] believed that part of the C₂H₄ generated at this time would undergo a polymerization reaction to form polyethylene, which would slow down the total volume increase of gas production.
The third stage is the high-voltage gas production stage, which mainly occurs at the positive end and produces a large amount of carbon dioxide (CO₂) and other gases. At this time, the slope of the gas production curve is equivalent to that of the first stage, and reaches the maximum at 3.647~3.671 V~ 365 μL. The fourth stage is the final stage of formation. As charging continues, the overall expansion volume of the battery cell no longer continues to increase, and shows a slight downward trend. This is mainly because a relatively stable SEI film has been formed on the surface of the positive and negative electrodes. The gas continues to be produced, but part of the C₂H₄ will still continue to undergo polymerization or consumption reactions with CO₂[4], resulting in a slight decline in the total volume of the cell in the later stage of formation (~16 μL). It is worth noting that the cell volume expansion detected by this device includes the soft expansion caused by gas production on the one hand, and the hard expansion caused by lithium ions intercalated into the Si/C negative electrode on the other hand, so the final result is the total volume of the cell volume change.
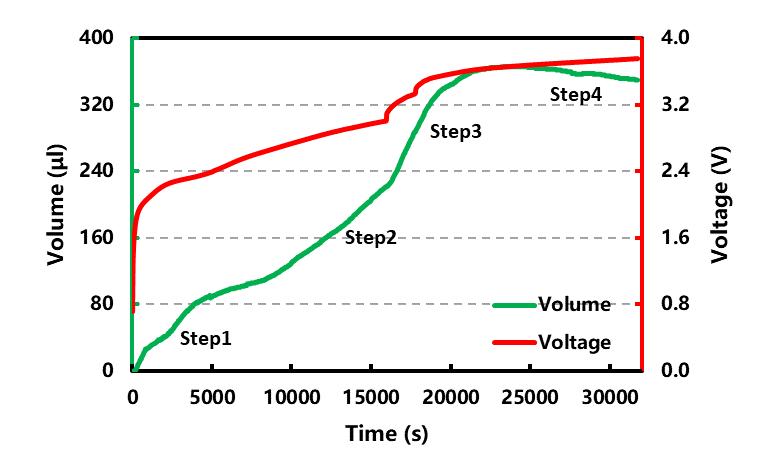
Figure 2. Battery charging curve and volume change curve
2.2 Results Analysis of Cell Expansion Behavior
Place parallel sample cell B of the same batch in in-situ swelling analyzer (SWE2110), set the pressure mode to constant pressure mode (the pressure value is constant at 5.0kg), and monitor the thickness of cell B in the entire formation process in real time changes, and the results are shown in Figure 3.
In the early charging stage of formation, the thickness change of cell B (green line) is not obvious, and there is even a slight decrease (-0.7μm). The Si/C negative electrode is not embedded to cause hard expansion, and the in-situ swelling analyzer (SWE2110) exerts a preload on the cell in the longitudinal direction, so that the gas generated by the formation tends to diffuse laterally into the air pocket on the side of the cell. The thickness change in the longitudinal direction was not caused (the thickness of the SEI film formed at the initial stage of formation is less than 1nm[7], and its influence on the thickness can be ignored), but the volume shrinkage caused by the delithiation of the positive electrode makes the relative thickness change curve below ~3.47 V There is a slight drop in the voltage range of .
When charging above ~3.47 V, a large amount of lithium ions are not only used for film formation, but also start to intercalate into the Si/C negative electrode and cause hard expansion. During the process, it increased rapidly until the end of the experiment. Therefore, the device and method mainly detect the hard expansion behavior of the battery core.
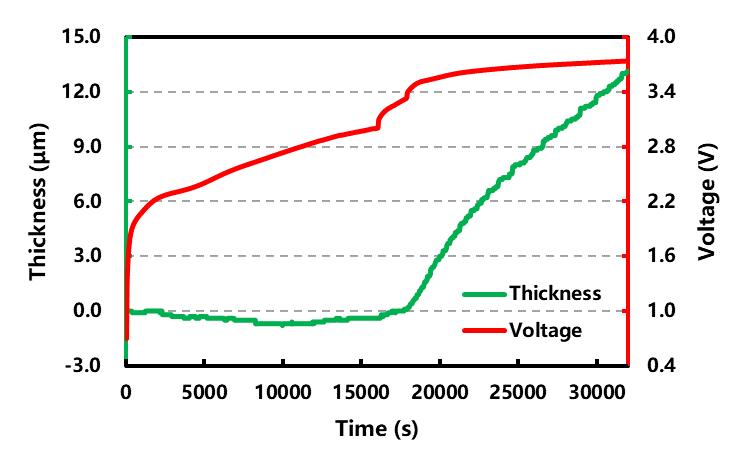
Figure 3. Cell charging curve and thickness expansion curve change with time
2.3 Separation of Cell Soft/hard Expansion
Since the test current is small, we ignore the influence of the concentration gradient of lithium ions on the uneven expansion of the cell thickness during charging. The large surface size of the pole piece of this batch of batteries is 60mm*45mm, and the volume of the battery during the hard expansion process can be obtained by multiplying the thickness change of the lithium de-intercalation of the battery (the test result in Figure 3) by the area of the pole piece Variety. Subtracting the volume change of the hard expansion of the battery cell from the total volume change (test results in Figure 2), the volume of gas produced during the formation process of the battery cell can be obtained, so as to effectively separate the soft expansion and hard expansion of the battery core. The result As shown in Figure 4.
It can be seen that in the voltage range before ~3.47 V, the proportion of soft expansion behavior (purple line) has been maintained at about 100% (the proportion is slightly greater than 100% because the cells not only have no obvious hard expansion behavior in the early stage of formation, but Instead, the delithiation of the cathode induces a subtle structural contraction, as described in Section 2.2), while above ~3.47 V, lithium ions begin to intercalate effectively into the Si/C anode, at which point the hard swelling behavior (orange line) It began to make a certain contribution to the total volume expansion of the battery, but when these contributions were the largest (at the end of the formation), they only accounted for about 10% of the total expansion.Therefore, the volume expansion of the cell during the entire formation stage mainly comes from the soft expansion behavior caused by gas production during film formation (accounting for more than 90%), while the hard expansion behavior caused by lithium intercalation mainly occurs in the middle and late stages of formation. , and the largest proportion is only about 10%.
Formation is mainly the process of forming a stable SEI film accompanied by gas production. Although the gas production in the later cycle of the battery is less, it is always accompanied by different degrees of gas production during the entire cycle, that is, the battery has a soft expansion process, especially in the process of Gas production is also obvious under conditions such as charging, over-discharging, and high temperature. The repeated accumulation of electrochemical expansion will also produce irreversible deformation. Therefore, during battery cycling or under safe test conditions, the method described in this paper can successfully distinguish and quantitatively characterize the soft/hard swelling of the battery, and provide a deeper analysis of the respective contributions of gas bulges and electrochemical swelling, thereby more A battery optimization strategy is proposed in a targeted manner.
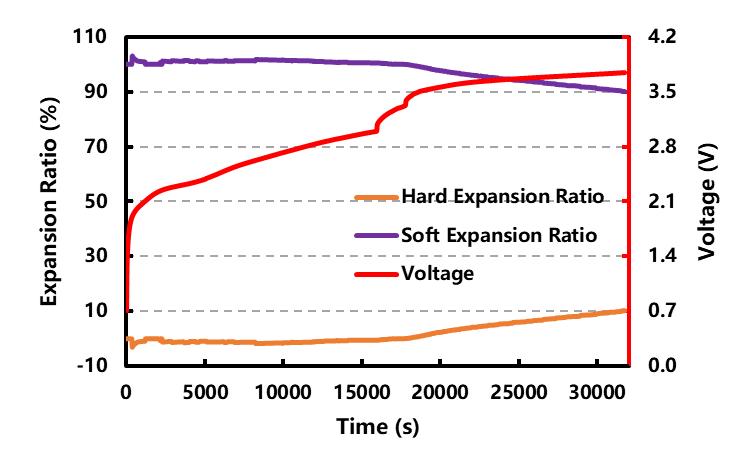
Figure 4. Cell formation expansion and gas production change curves
3. Conclusion
In this paper, the in-situ volume monitor (GVM) of Initial Energy Science&Technology Co.,Ltd. is used together with the in-situ swelling analyzer (SWE),Quantitatively characterize and separate the soft/hard expansion behavior of the NCM/SiC system cell during the formation stage, and find that throughout the formation stage, the total volume expansion of the cell mainly comes from the soft expansion caused by gas production during film formation Behavior (accounting for more than 90%), while the hard expansion behavior caused by lithium intercalation mainly occurs in the middle and late stages of formation, and when it accounts for the largest proportion, it is only about 10%.
The in-situ quantitative separation method is helpful for relevant technicians to conduct accurate and in-depth research on the expansion behavior of silicon-based negative electrode materials, and promotes the commercialization process of silicon-based negative electrodes.
4. References
[1] J.R. Dahn, Phase diagram of LixC6. Phys. Rev. B 44 (1991) 9170-9177.
[2] S. Chae, M. Ko, K. Kim, K. Ahn and J. Cho, Confronting issues of the practical implementation of Si anode in high-energy lithium-ion batteries. Joule 1 (2017) 47-60.
[3] J.N. Reimers and J.R. Dahn, Electrochemical and in situ X-ray diffraction studies of lithium intercalation in LixCoO2. J. Electrochem. Soc. 139 (1992) 2091-2097.
[4] J. Self, C.P. Aiken, R. Petibon and J.R. Dahn, Survey of gas expansion in Li-ion NMC pouch cells. J. Electrochem. Soc. 162 (2015) A796-A802.
[5] S.L. Guillot, M.L. Usrey, A. Pena-Hueso, B.M. Kerber, L. Zhou, P. Du and T. Johnson, Reduced gassing in lithium-ion batteries with organosilicon additives. J. Electrochem. Soc. 168 (2021) 030533.
[6] T. Yin, L.L. Zhang, L.Z. Jia, Y. Feng, D. Wang and Z.Q. Dai, Overview of research on float charging for lithium-ion batteries. Energy Storage Sci. Technol. 10 (2021) 310-318.
[7] Y. Wang, J.Q. Kang and Z.X. Tan, Study on SEI reaction of lithium-ion batteries based on the electrochemical degradation model. J. Chem. Eng. Technol. 8 (2018) 137-150.
