Characterization of Gas Generation Behavior at Different Silicon-oxygen Material Slurry Levels
In recent years, people have placed higher and higher requirements on battery life, safety, fast charging and other performance. Silicon-based negative electrode materials are new high-performance lithium-ion battery negative electrode materials due to their advantages such as high specific capacity, good safety, and abundant sources. With the development of high-capacity silicon-based anode materials (such as nano-silicon carbon and silicon oxide carbon anode materials), methods such as surface modification and element doping have been widely used to improve material performance. Among them, pre-lithium technology significantly improves first efficiency and battery energy density. However, technologies such as surface modification and element doping are often accompanied by unstable factors, such as surface alkalinity and incomplete coating, which can cause nano-silicon to be exposed and react with hydroxyl ions to generate gas during the pulping process. In practical applications, silicon-based negative electrode materials generally use water as a solvent for homogenization, but it easily reacts with water to cause the slurry to generate gas, thus affecting the quality of subsequent coating processes.
This article uses IEST's In-Situ Gassing Volume Analyzer (GVM2200) to monitor the gas production of each modified material to achieve rapid screening of modification processes on the material side.
1. Testing Equipment
1.Testing Equipment: In-Situ Gassing Volume Analyzer, model GVM2200 (IEST), which can adjust the temperature from 20℃ to 85℃. The equipment appearance is shown in Figure 1.

Figure 1. Schematic diagram of In-Situ Gassing Volume Analyzer
2. Test Plan and Pre-processing
In this experiment, aluminum plastic film was used to encapsulate the sample and a counterweight was added (as shown in Figure 2 below). Combined with GVM2200 equipment, the drainage method was used, and Newton's theorem and Archimedes' buoyancy law were used, the quality change of the sample slurry over time is measured in real time by a high-precision sensor, and is further converted to obtain the change in gas production volume.

Figure 2. Sample Preparation Process
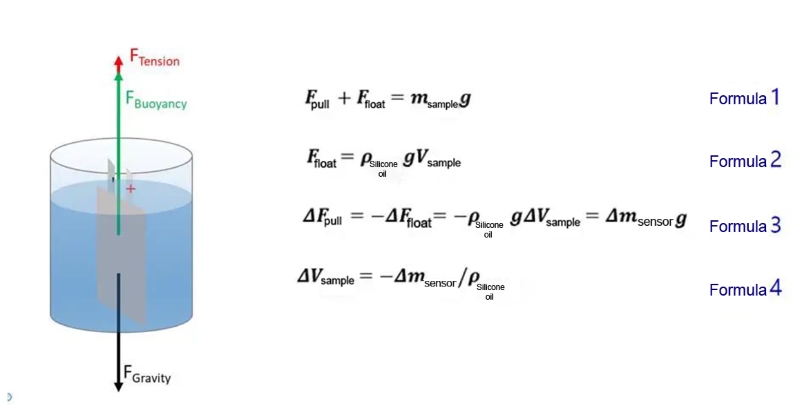
Figure 3. Test Principle Diagram
2. Test Process and Results Discussion
In response to the problems of low first discharge efficiency, poor conductivity, and poor cycle performance of silicon-based anode materials, a large amount of research on prelithiation technology has been conducted. By adding a small amount of lithium source in advance to supplement the lithium consumed inside reactions and solid electrolyte interface (SEI) film formation, it is further improved by combining surface modification and other solutions. On the one hand, the prelithiation agent is sensitive to water and easily reacts to produce gas; on the other hand, research shows that elemental silicon is also easily hydrolyzed, producing flammable hydrogen as a by-product:
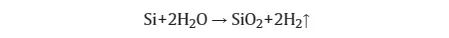
Surface coating is a common surface modification method, which can be divided into single-layer core-shell structure, multi-layer core-shell structure and sandwich structure. The single-layer core-shell structure uses silicon particles as the core and is coated with a layer of conductive carbon material on the periphery. The silicon provides high capacity, and the peripheral carbon material can improve the conductivity and slow down the expansion of silicon, thereby improving cycle performance. The multi-layer core-shell structure is coated multiple times on the basis of the single-layer core-shell structure. Effective synergy is formed between the multi-layer coating layers to more effectively prevent the silicon particles from being exposed.
Three different modified silicone materials were selected (sample #1 is a "pre-lithiation + carbon source secondary coating" process, sample #2 is a "pre-lithiation + carbon source secondary coating" process), sample 3# is a "pre-lithiation + B carbon source one-time coating" process). After being mixed with deionized water at a ratio of 1:6, it is packaged and pre-treated as shown in Figure 2, the gas production over time at a high temperature of 60°C is shown in Figure 4 below: as time prolongs, the gas production of the three modification processes gradually increases. Comparing samples 1# and 2# shows that different carbon sources have different coating effects on silicon-oxygen substrates. Comparing samples 2# and 3#, the gas production rate is similar in the early stage and increases sharply in the later stage, indicating that multiple coating processes can significantly improve the integrity of the material coating.
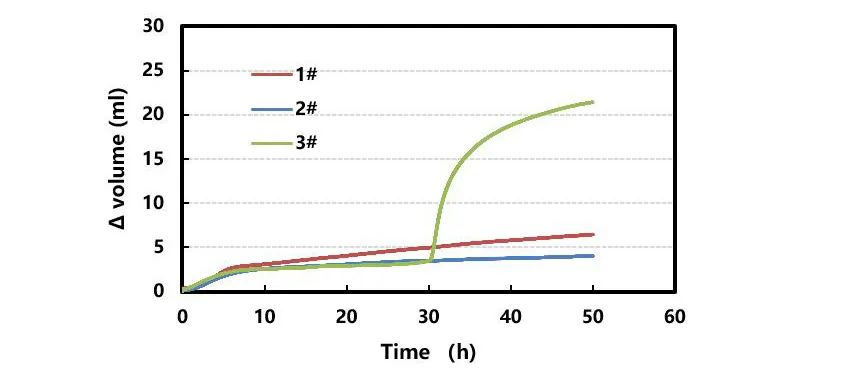
Figure 4. Effects of different modification conditions of silicon carbon powder on gas production (60°C)
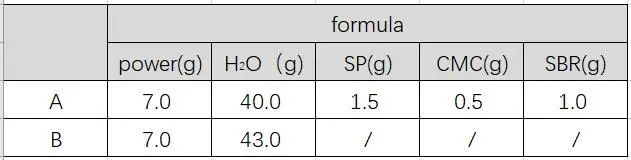
In the actual pulping process, conductive agents, binders, etc. are usually added, and then the final slurry is obtained by combining the dispersion process. To compare the difference in gas production between the samples in the formula and pure water environment, the slurries were prepared according to the proportions in Table 1 below, and their gas production at different temperatures were monitored respectively.
Table 1. Slurry Ratio
As shown in Figure 3: The gas production of the slurry of the same formula increases as the test temperature increases. This may be related to the temperature increase accelerating the gas production reaction between the sample and hydroxide radicals. At the same time, it was also found that under different temperature conditions, the gas production amount showed that the A formula slurry was more than the B formula slurry. This may be mainly because the binder has a catalytic effect on the gas production process, thus increasing the gas production rate and volume.
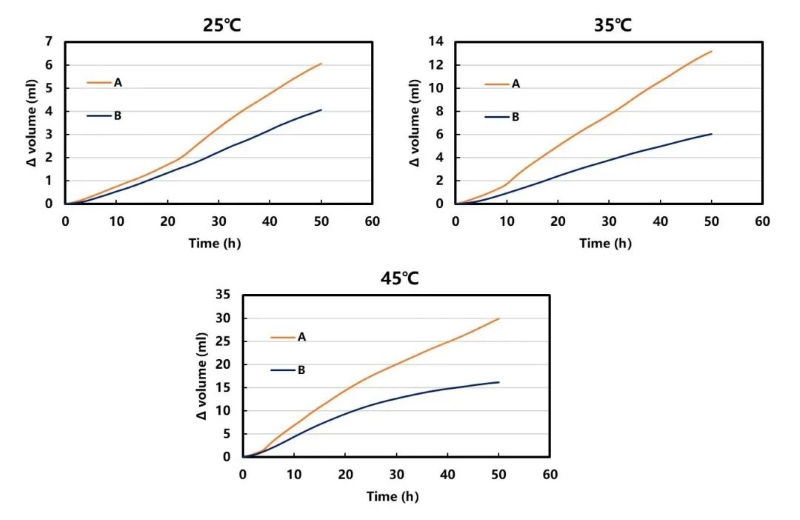
Figure 5. Effect of different slurry ratios on gas production
Research in literature [1] shows that lithiated polycarboxylate (such as LiPAA) binders have a catalytic effect on gas evolution when present in solution. As shown in Figure 6a, the effects of water and NMP on gas production are first compared. The results show that Si particles in NMP produce negligible gas, while Si particles in water produce significant gas. Figure 6b shows that the higher the degree of lithiation, the larger the gas volume generated by the LiPAA aqueous Si slurry. In reaction (1), the theoretical hydrogen production amount per gram of silicon is 1.7L. However, a hydrogel-like silica layer will form on the surface during the initial stage of silicon hydrolysis, which slows down the diffusion of water, resulting in a much lower hydrogen yield than theoretically. On the other hand, any instability of the protective layer may lead to rapid consumption of silicon and gas production. This instability usually occurs in alkaline solutions (an environment where silicates can dissolve), this is mainly because these alkalinities increase the solubility and porosity of the silica hydrogel. According to the results of this study, for CMC-Na, due to Na substitution, it may also have a catalytic effect on Si gas production. Therefore, when the binder is added to the slurry, the gas production of the sample increases significantly.
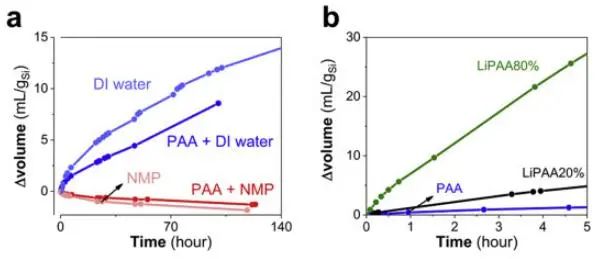
Figure 6. (a) Slurry of Si and deionized water or NMP without or with 2.5 wt% PAA and (b) gas volume change of aqueous Si slurry containing PAA and LiPAA with different lithiation degrees (the percentage in the figure represents the degree of lithiation)
3. Summary
This article uses In-Situ Gassing Volume Analyzer (GVM2200) to characterize the impact of different modification processes on the gas production of silicon-based anode slurries. It also clarifies the impact of different formulas and different temperatures on the gas production of the slurry. It can provide an effective verification method for the verification of the modification effect of silicon-based anode materials and can also provide a direction for the improvement of the homogenization process.
4. Reference Literature
[1] Rodrigues M F, Trask S E, Shkrob I A, et al. Quantifying gas generation from slurries used in fabrication of Si-containing electrodes for lithium-ion cells[J]. Journal of Power Sources, 2018, 395(AUG.15): 289-294.DOI: 10.1016/j.jpowsour.2018.05.071.
