Effect of Charge-discharge Ratio on Volume Expansion in Resting Stage
Lithium-ion batteries are accompanied by volume swelling in the process of formation, circulation, storage and overcharging process, including structural swelling and gas production swelling. The charge and discharge rate determines the rate of the lithium removal reaction of the cell, which is also accompanied by different degrees of heat production or lithium precipitation. When studying the electrical performance of the cell, researchers usually increase a certain period of time at the end of charging or discharge to stabilize the state of the cell and eliminate the thermal effect or polarization. How does the volume of the cell change during the resting stage? This paper explores the effect of multiplier on the swelling of LCO / Graphite system.
Figure 1. Various factors affecting the fast charging of lithium-ion batteries[1]
1.Test information
1.Test Equipment: In-situ gas production volume monitor, model GVM2200, can control the temperature of 20℃ ~80℃, as shown in Figure 2.

Figure 2. In-situ gas production volume monitor
2. Test Parameter
2.1 The Cell Information is Shown in Table 1
Table 1. Cell Information
Cell Information | |
Material | LCO/ Graphie |
Capacity | 2000mAh |
Voltage | 2.8~4.35V |
Model | Pouch cell-345877 |
3. Experimental Parameters
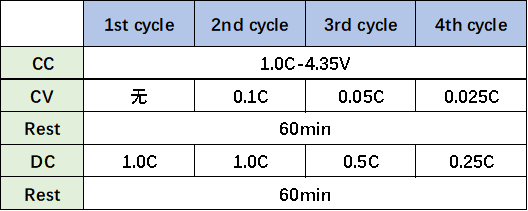
Four experimental groups are set according to the parameters in Table 2 below, and shelved for 1 hour after each charging and discharge. The battery is then placed in the in-situ gas production volume monitor, adjust the oil bath temperature to 25℃, and monitor the volume change of the cell in real time.
Table 2. Charge and Discharge Parameters
4.Interpretation of Result
4.1 Monitor In-situ the Whole-process Cell Volume Swelling Curve
The charge-discharge curves and the volume change curves for the four cycles are shown in Figure 3. Constant current charging stage: As the S OC increases continuously, the volume expands continuously, which is mainly related to the structural expansion of lithium-ion constantly embedded in graphite. After entering the constant pressure and shelved stage, the volume of the cell began to shrink and gradually reached stability. In the constant current discharge stage: with the gradual increase of DOD, the volume of the cell cell continues to shrink, and when the doubling rate of the discharge gradually becomes small, the abnormal swelling of the initial volume of the cell discharge is also gradually decreasing. Entering the shelved stage after discharge, the volume of the cell continues to decrease and stabilize with time. Next, we perform a detailed analysis of the shelving stages A and B after charging and discharge.
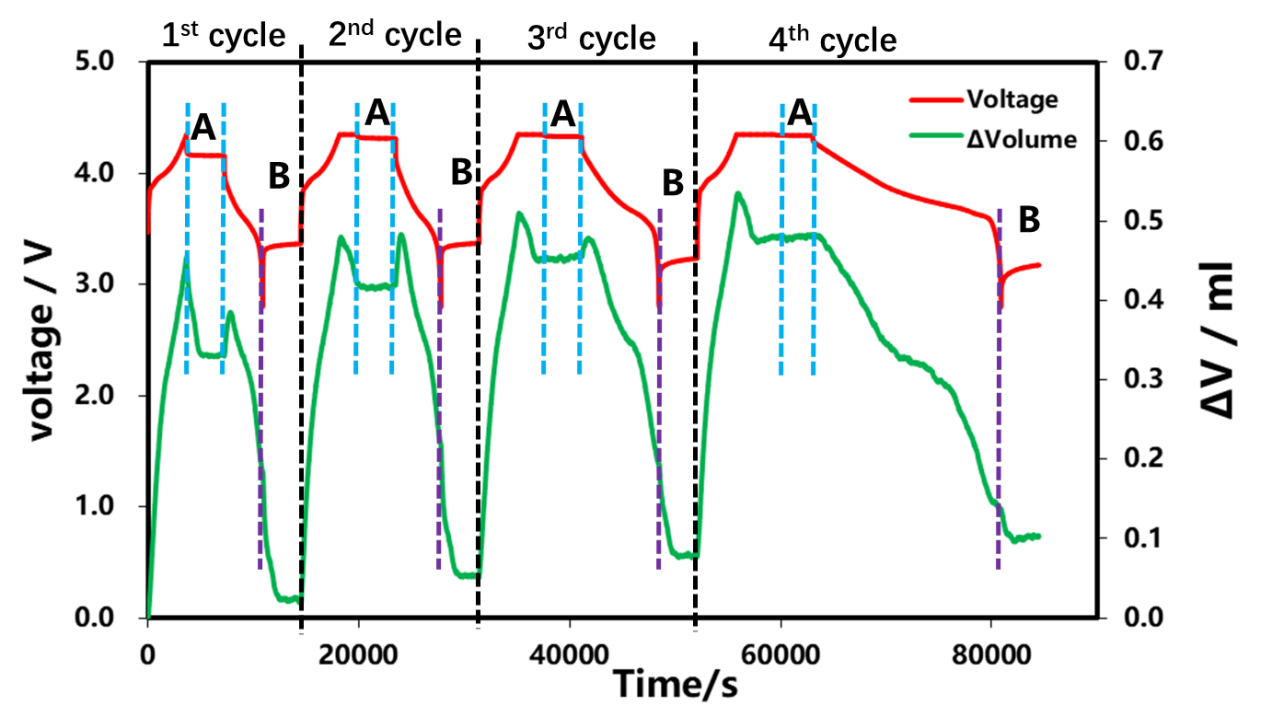
Figure 3. Different charge and discharge current and volume change curve of electric cells
4.2 Volume Swelling Analysis in the Charging Shelved Stage
The rechargeable shelved part A of each lap in Figure 3 was selected for analysis, as shown in Figure 4. As the cut-off current of each lap is gradually reduced, according to the shelved volume curve, when there is no C V in the first lap, the cut-off current of charging is 1C. At this time, the volume gradually decreases with the shelving time and remains unchanged after about 1500s. In the last three circles, the constant pressure stage is increased. When the charging cut-off current is less than 0.1C, the volume contraction appears at the beginning of the constant pressure, and then when the shelved after charging is increased, the volume is basically unchanged. This shows that volume shrinkage and current reduction, when there is constant pressure, current reduction accompanied by volume shrinkage, this is likely to be constant voltage stage battery internal polarization reduction, lithium concentration difference in electrode thickness direction, lithium concentration difference stress and strain reduction if no constant pressure stage, at the end of charging, the battery internal polarization, lithium concentration difference is also very big, shelved stress and strain release, at least half an hour to achieve stable state.
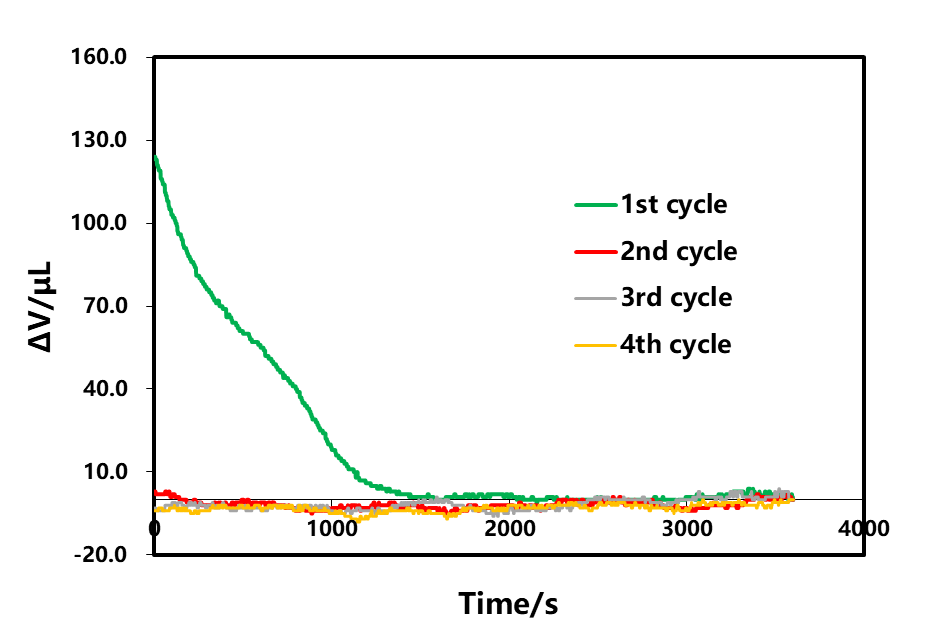
Figure 4. Effect of the charge cut-off current on the shelved volume
In order to further explain the process of lithium-ion transmission and charge state evolution in the constant current-constant pressure charge process, the mechanical-electrochemical model is used to study the distribution of lithium concentration, stress and strain in the charging process.
4.3 Constant-current Charging Stage
During the constant flow charging phase, lithium-ion are continuously embedded in the graphite layer, which leads to structural swelling. As the SOC continuously grows, the volume constantly expands. When the constant current charging is completed, the lithium ion concentration in the electrolyte, the lithium concentration in the negative electrode particles, the strain, and the stress are as shown in Figure 5. Figure 5 (a) shows that the closer the cathode fluid collection is, the higher the lithium-ion concentration in the electrolyte is. This is because the positive / negative particles near the diaphragm are preferentially lithium / lithium. At the end of constant current charging, the degree of lithium removal / lithium removal of the particles near the diaphragm is greater than that of the particles near the fluid collection. From FIG. 5 (b), higher lithium concentration in the negative particles close to the diaphragm. This is because the electrolyte in the negative region away from the diaphragm has a longer lithium diffusion distance than the negative particles near the diaphragm, allowing the lithium-ion reaching the anode to be preferentially inserted into the particles close to the diaphragm.
In the negative electrode particles near the diaphragm, the solid-phase lithium concentration is higher, so their strain is also higher, as shown in Figure 5 (c). Meanwhile, the strain is greater on the free surface of the particles, but less so on the contact surface between the particles and between the particles and the boundary. This is because the insertion of lithium ions causes the volume swelling of the particles. The free surface of the particles expands outward, leading in greater deformation. However, the deformation on the contact surface is smaller under the constraints of adjacent particles and boundaries. Figure 5 (d) shows that the stress distribution is opposite to the strain distribution. This is because the free surface of the particles is unconstrained and therefore stress less. However, the contact surface is strongly constrained and therefore greater stress. The maximum stress is located in the particles close to the diaphragm. The average strain of the negative electrode particle (meaning the mean value of the whole body of the particle in the electrode) during the constant current charging is shown in Figure 6. Both increase gradually with time, and their change trend is basically consistent with the relationship shown in Figure 3. After CC charging, the average strain and average stress reach the maximum value respectively.
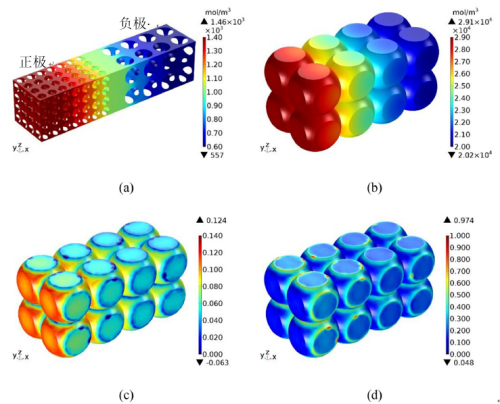
Figure 5. Lithium-ion concentration (a) and graphite particle solid phase lithium concentration (b), strain (c) and stress (d) distribution of graphite particles at the end of 1C CC charging[2]
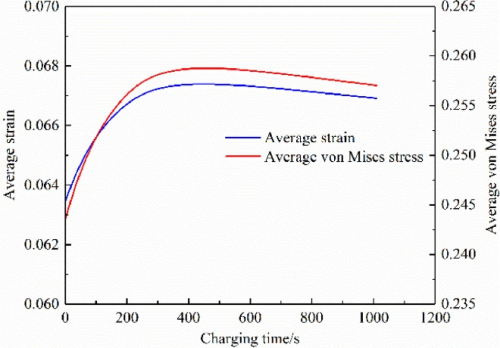
Figure 6. Average strain and average stress of the anode particles during C C C charging[2]
4.4 Constant Voltage Charging Stage
After the constant current charging is completed, the constant voltage is charged at the cut-off voltage until the current drops to the cut-off current. The lithium ion concentration in the electrolyte and the lithium concentration, strain and stress in the negative electrode particles at the end of the constant pressure charging are as shown in Figure 7. Figure 7 (a) shows that the lithium ion concentration in the electrolyte is the same in almost all regions. Comparing Figure 7 (a) and Figure 5 (a) shows that the maximum lithium-ion concentration in the electrolyte at the end of CV charging is lower than the maximum lithium-ion concentration at the end of CC charging. This is because the current is still high at the end of the CC charging, so large amounts of lithium ions still emerge from the positive electrode. But at the end of the CV charging, the current is close to 0, so very few lithium-ion emerge from the positive electrode.
It can be seen from Figure 7 (b) that despite the CV charging, the lithium concentration in the negative electrode particles remains uneven at the end of the charging. The concentration of lithium was higher in the particles close to the diaphragm. This is because the current has not dropped to 0 at the end of the CV charging. However, a comparison between FIGS. 7 (b) and Figure 5 (b) shows that the lithium concentration difference in the negative electrode particles at the end of CV charging is significantly lower than the lithium concentration difference at the end of CC charging. If the CV charging time is extended, that is, the cut-off current is continuously reduced, the lithium concentration difference in the negative electrode particles will continue to decrease to a negligible degree. Comparing FIG 7 (b, c) and 5 (b, c) shows that the lithium concentration and strain in the negative electrode particles at the end of CV charging are more uniform than at the end of CC charging. This means that the polarization effect is alleviated during CV charging. Comparing FIG 7 (d) and 5 (d) shows that the stress in the negative electrode particles at the end of CV charging is greater than the stress at the end of CC charging. This is mainly because the lithium-ion are further embedded in the particles during the CV charging process.
Figure 8 shows the average strain and average stress of the negative electrode particles during CV charging. It can be found that they first increase with time, and then both show a small downward trend. This is mainly because the lithium-ion are further embedded into the particles during the CV charging process. But the difference in lithium concentration in the particles is getting smaller and smaller. Therefore, the strain and stress caused by the lithium concentration difference decrease. The simulation results are consistent with the experiments shown in Figures 3 and 4.
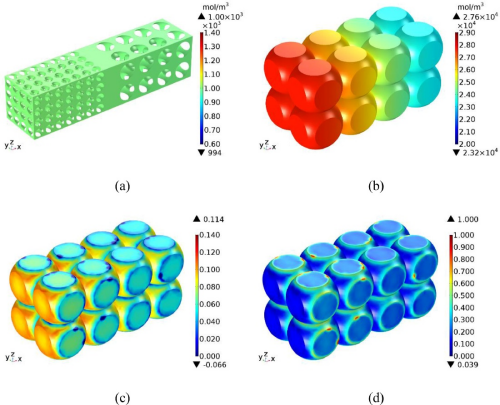
Figure 7.Lithium-ion concentration (a) and negative particle solid phase lithium concentration (b), strain (c) and stress (d) distribution in the electrolyte at the end of C V charging[2].
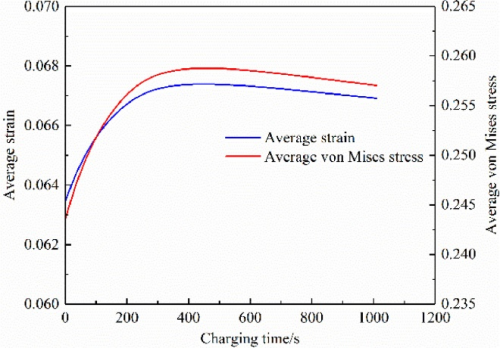
Figure 8. Average strain and average stress of the negative electrode particles during the C V charging period[2]
4.5 Volume Swelling Analysis of Discharge and Shelved Stage
The discharged shelved part B of each loop in Figure 3 was selected for analysis, as shown in Figure 9. From the perspective of the volume curve, as the rate of discharge rate decreases, the volume change is gradually reduced, and the time of volume stability is gradually shortened. The phenomenon of volume shrinkage in the shelved stage after discharge is similar to the shelved stage after charging, which is related to the polarization and uneven lithium concentration distribution inside the cell. The volume change curve corresponding to different multiplier discharge is analyzed. As shown in Figure 10, in the initial stage of discharge, the volume of the cell is abnormally expanded, and with the decrease of the discharge ratio, the swelling amount also gradually decreases, which is likely to be related to the internal thermal effect of the cell caused by the large multiplier.
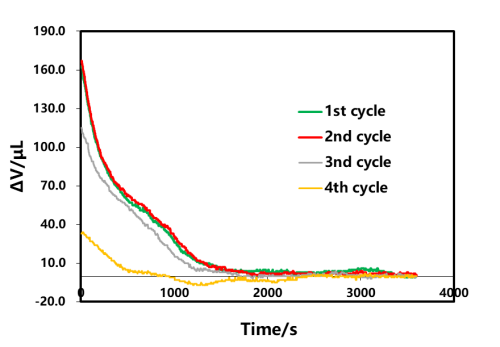
Figure 9. Effect of discharge current on shelved volume
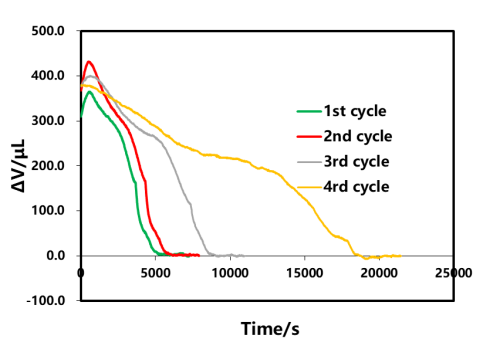
Figure 10. Effect of the discharge current on the total volume and the rebound volume
According to the simulation results of the constant current and constant voltage charging process, the lithium is disembedded from the negative electrode particles as the discharge process continues. At the same time, the electrochemical potential drives the lithium in the electrolyte by moving from the negative electrode to the positive electrode. The lithium concentration in the negative particles decreased but increased in the positive particles. In the direction of electrode thickness, there is also the problem of uneven lithium concentration distribution. This difference of lithium concentration is related to the thickness and multiplier of the electrode. As shown in FIG 11, at the end of the discharge, the electrode thickness is relatively small or the doubling ratio is relatively small, and the lithium concentration distribution is relatively uniform. When the electrode thickness or multiplier increases, a significant concentration difference appears.For the positive pole, particle lithium concentrations near the diaphragm and close to the fluid collector are low.Therefore, with the decrease of the discharge rate, the difference of lithium concentration within the battery becomes smaller, and the stress and the change caused by the concentration difference should be small. The amount of volume change is also gradually reduced, and the time of volume stability is gradually shortened.
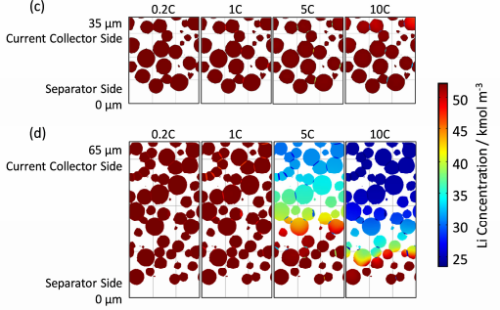
Figure 11. Grain solid-phase lithium concentration distribution at the end of the discharge[3]
5.Summarize
This paper adopts the in-situ gas volume monitoring instrument (GVM2200), monitoring the LCO / Graphite system cell volume swelling behavior at different rates, found that the process of volume swelling behavior is not only related to the embedded lithium behavior, but also related to the charge and discharge lithium concentration distribution, may also be related to the cell thermal effect caused by current. Therefore, setting the appropriate charging cut-off current can effectively eliminate the impact of the uneven distribution of lithium concentration inside the cell on the volume swelling. However, due to the discharge process current is generally large, the corresponding increase of shelved time after the discharge is needed to make the cell reach a stable state.
6.Reference Documentation
1. Anna Tomaszewska, Zhengyu Chu, Xuning Feng, et al.Lithium-ion battery fast charging: A review,eTransportation, 1 (2019) 100011.
2. Factors affecting stress in anode particles during charging process of lithium ion battery, Journal of Energy Storage, 43(2021)103214.
3. Hideki Kikukawa, Kohei Honkura, Michihisa Koyama.Influence of inter-particle resistance between active materials on the discharge characteristics of the positive electrode of lithium ion batteries, Electrochimica Acta, 278(2018)385-395.
